Almond is a professional, cloud-based, quality management software for healthcare companies.
The software enables computerized management of the quality processes in compliance with regulatory requirements.
The system maintains a document audit trail, full document indexing and ultimately achieves documented evidence of all QMS processes.
The system maintains a document audit trail, full document indexing and ultimately achieves documented evidence of all QMS processes.
regulations
- 21 CFR Part 11
- ISO 13485:2016
- 21 CFR Part 820
Almond Modules
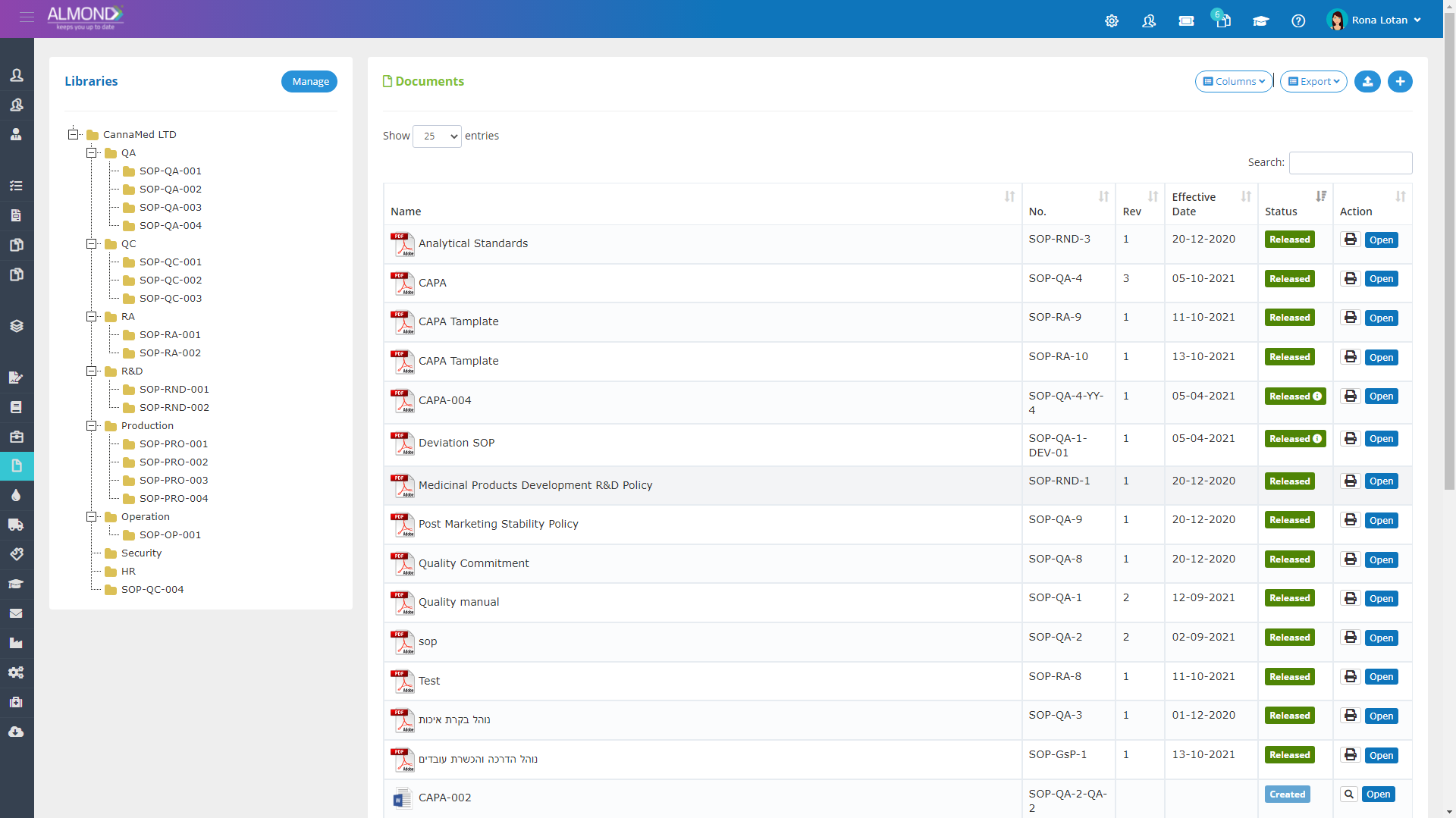
An intuitive and simple document management module:
Personalized folder structure for each client
Document management without space limitations
Simplified document sharing
Saving version history
Dashboard Control permissions for users
Automatic updating of document details
Personal electronic signature for each user
Automatic conversion to effective after training completion
Restrict use of obsolete revisions
Automatic mail notification of document status
Automatic documentation of document changes
Documentation of approval & reviewers history

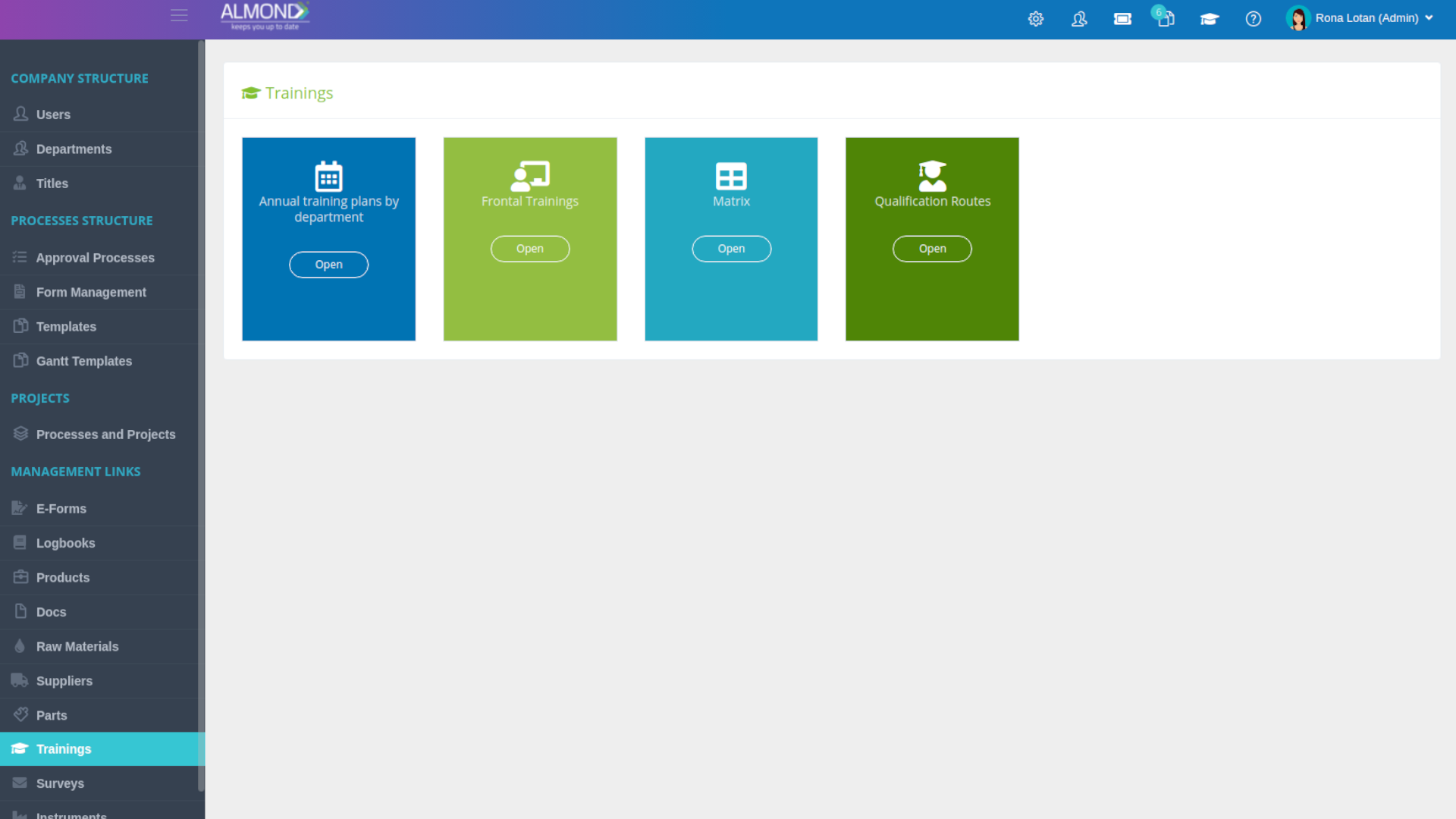
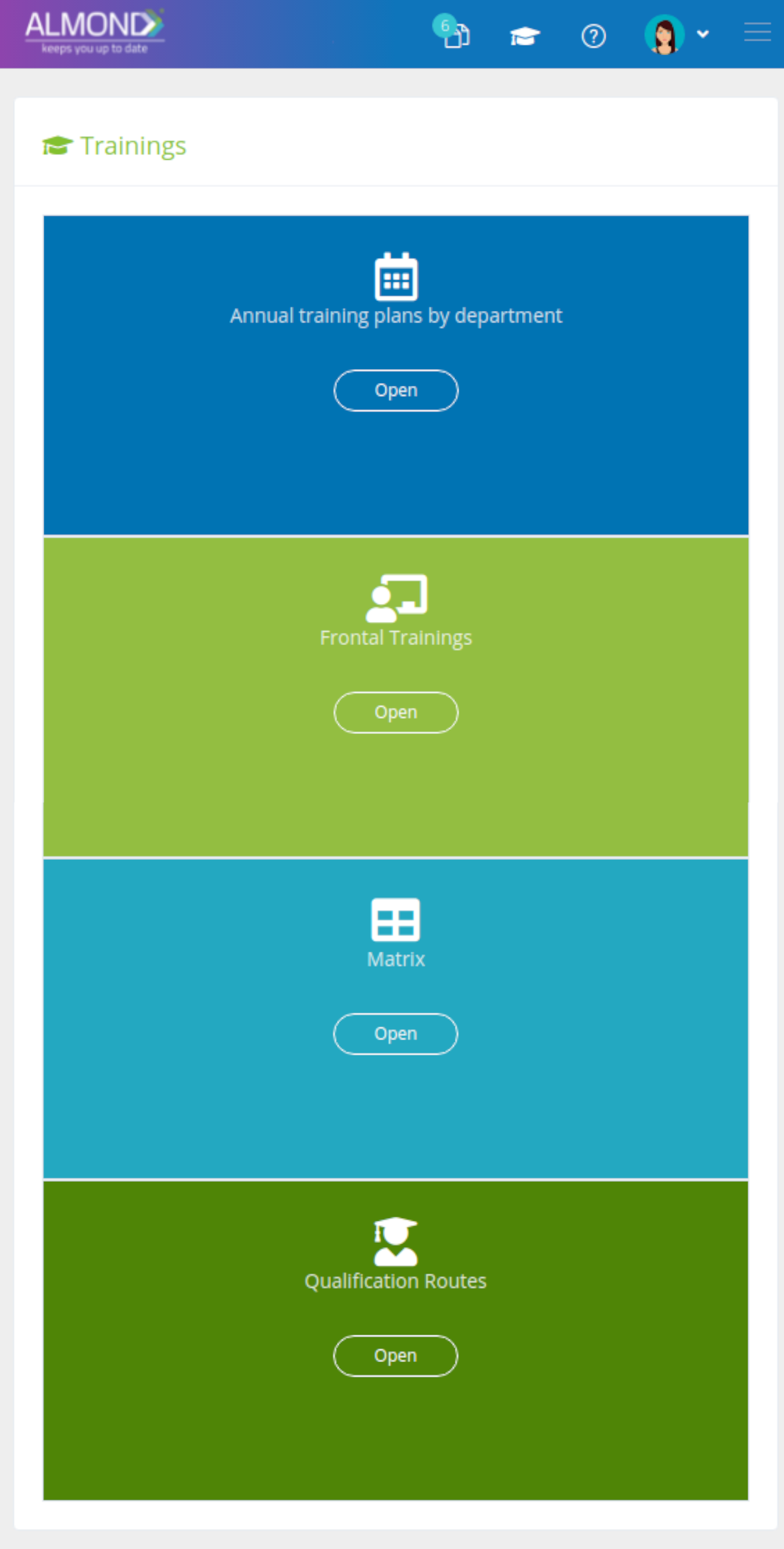
An easily and simple training management module:
Personalized folder structure for each client
Document management without space limitations
Simplified document sharing
Saving version history
Dashboard Control permissions for users
Automatic updating of document details
Personal electronic signature for each user
Automatic conversion to effective after training completion
Restrict use of obsolete revisions
Automatic mail notification of document status
Automatic documentation of document changes
Documentation of approval & reviewers history

An intuitive and simple document management module:
Personalized folder structure for each client
Document management without space limitations
Simplified document sharing
Saving version history
Dashboard Control permissions for users
Automatic updating of document details
Personal electronic signature for each user
Automatic conversion to effective after training completion
Restrict use of obsolete revisions
Automatic mail notification of document status
Automatic documentation of document changes
Documentation of approval & reviewers history

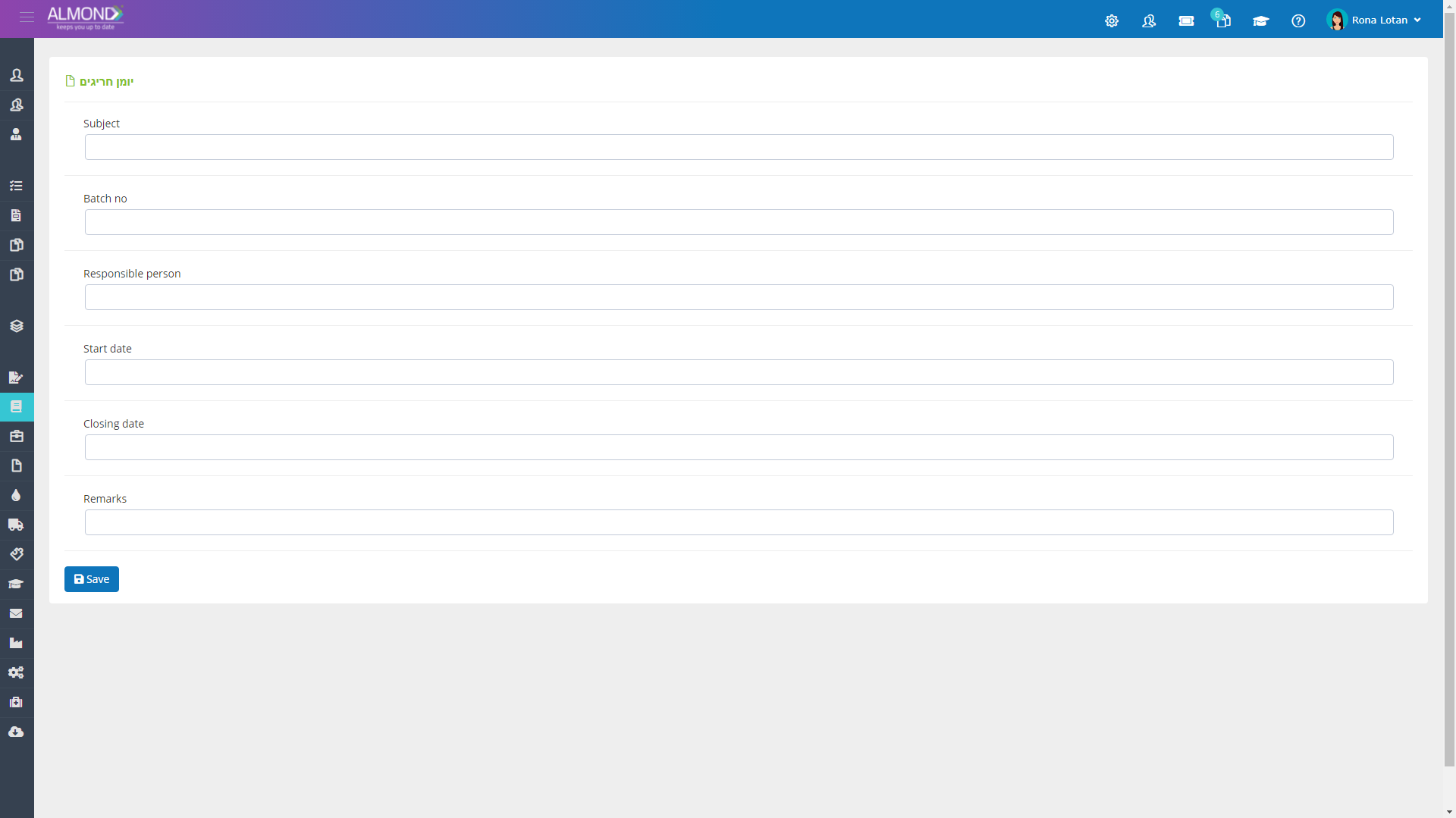
Manage your work records in a convenient and accessible electronic logbook:
Improve the efficiency of your work and eliminate the worry of “losing” the knowledge of where it is documented.
Set your logbook's columns once, and add rows below as needed
Set places for you to sign electronically, If necessary
Create as many logbooks as you need, with no budget considerations or storage restrictions
Turn your logbook into a management tool that enables task management
Export your logbooks to Excel, Word, or pdf
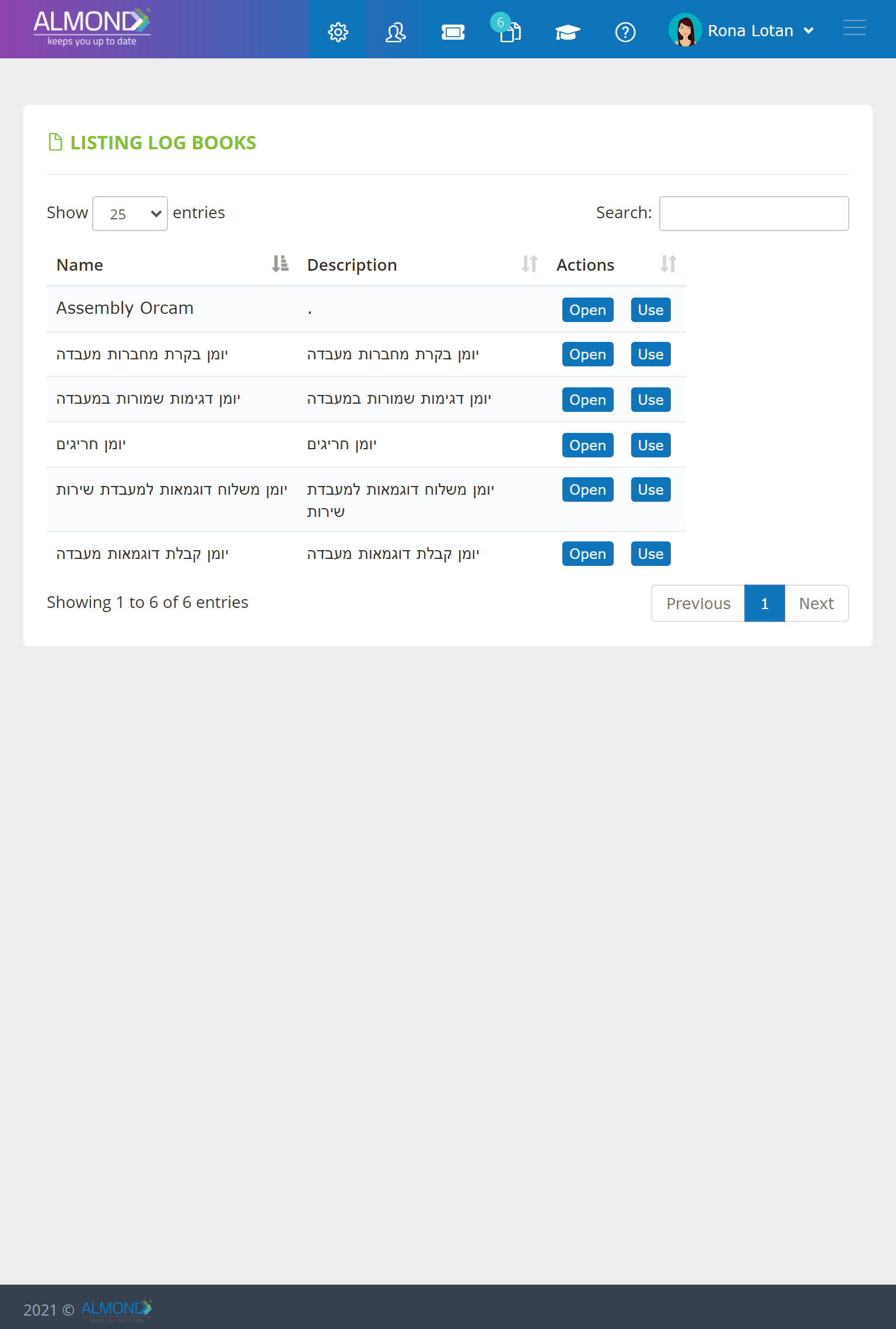

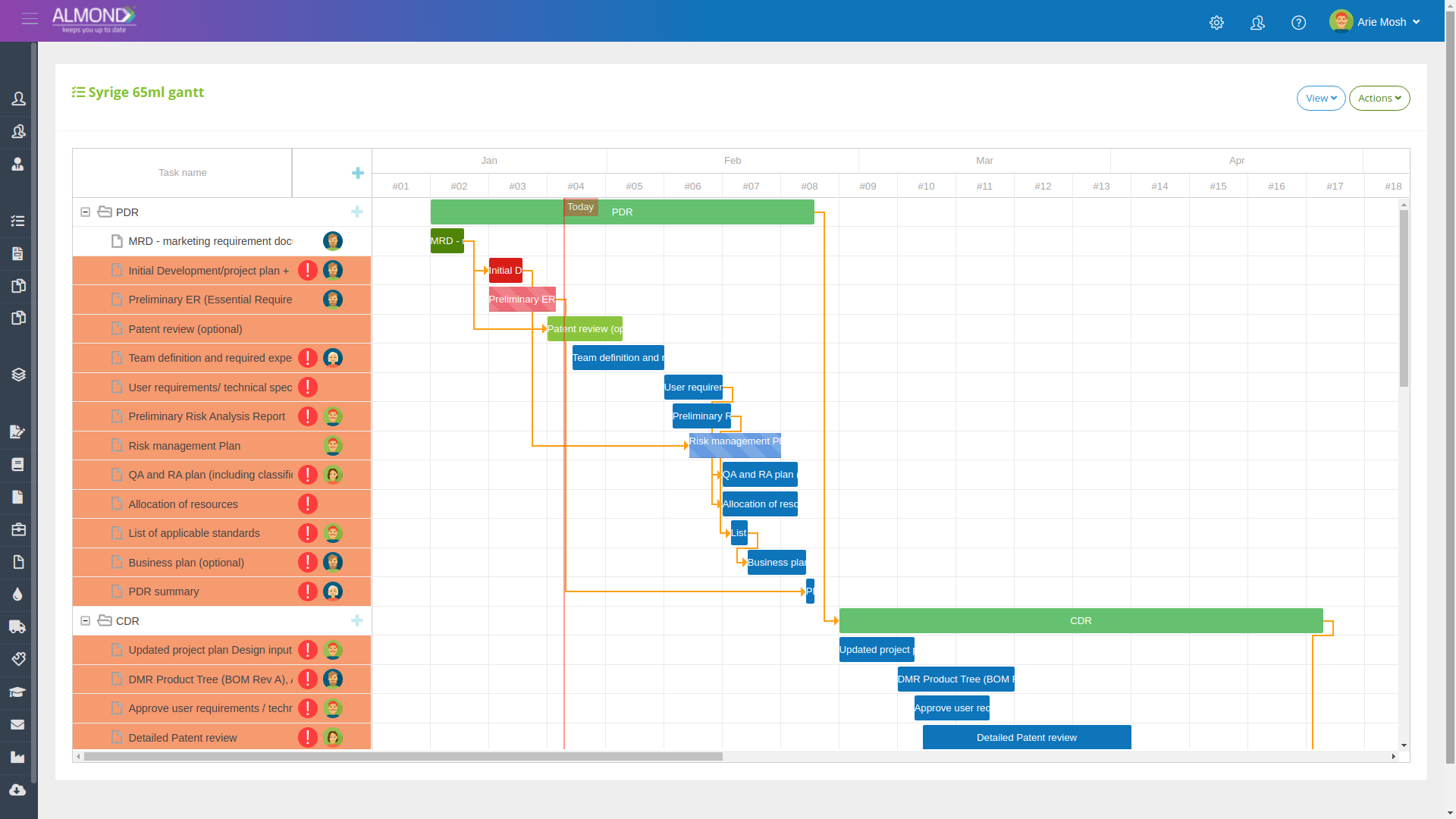
Manage hybrid projects, in an advanced application:
Insert tasks into a clean timeline
Define responsible parties for tasks
Automatically distribute tasks to team members
An electronic control dashboard allows you to be updated at any time on the status of the project

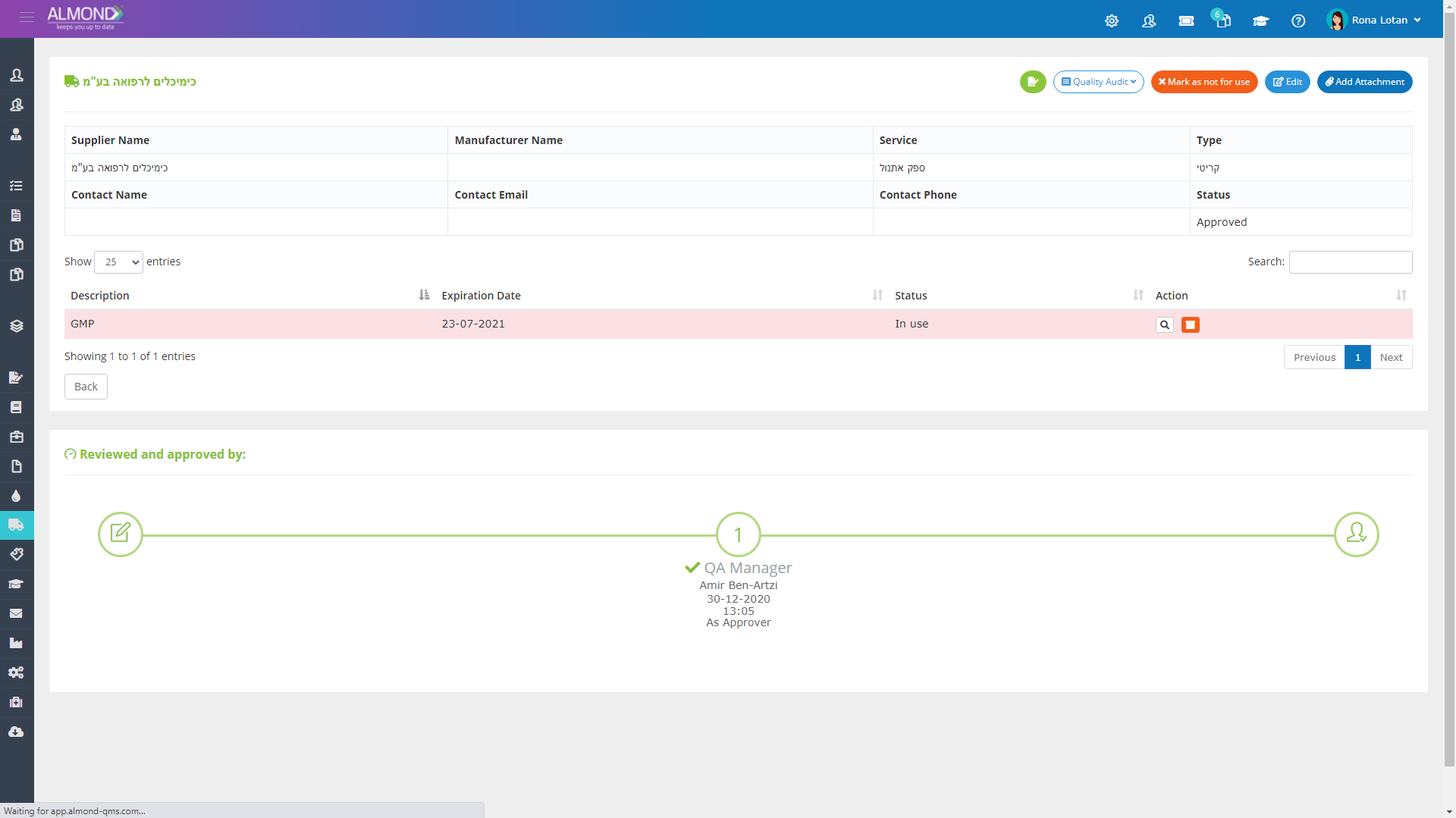
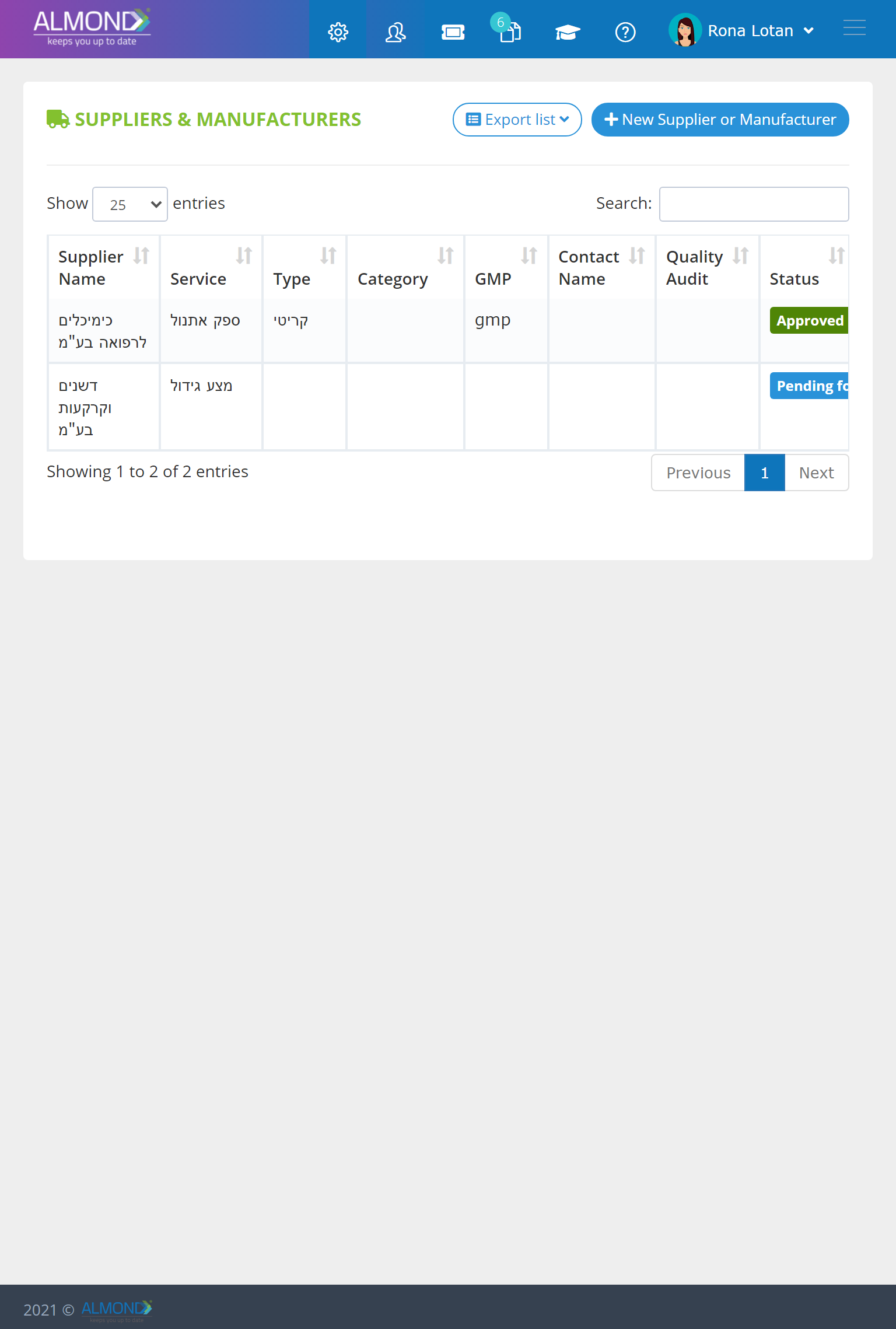
Manage your suppliers seamlessly: Electronic organization of certificates and agreements- All information concentrated in one place
Define critical suppliers and assign risk ratings
Supplier quality audit management
Differentiate approved and non-approved suppliers
Assign permission to your suppliers to perform training, required by your system at their premises, using your quality system
Receive automatic email notifications before supplier certification expires
Assign permission to one or more of your employees to be in charge of supplier management.

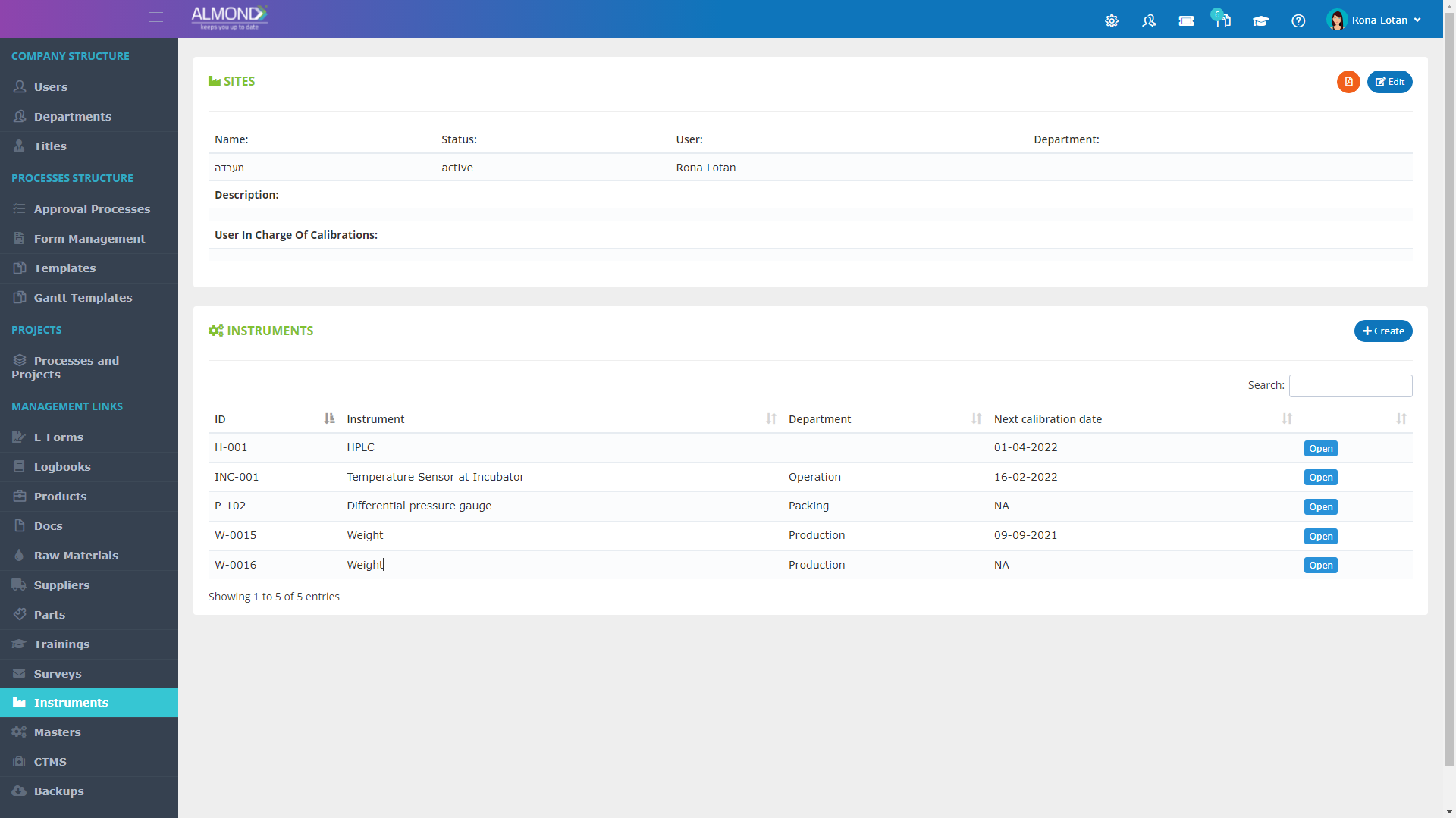
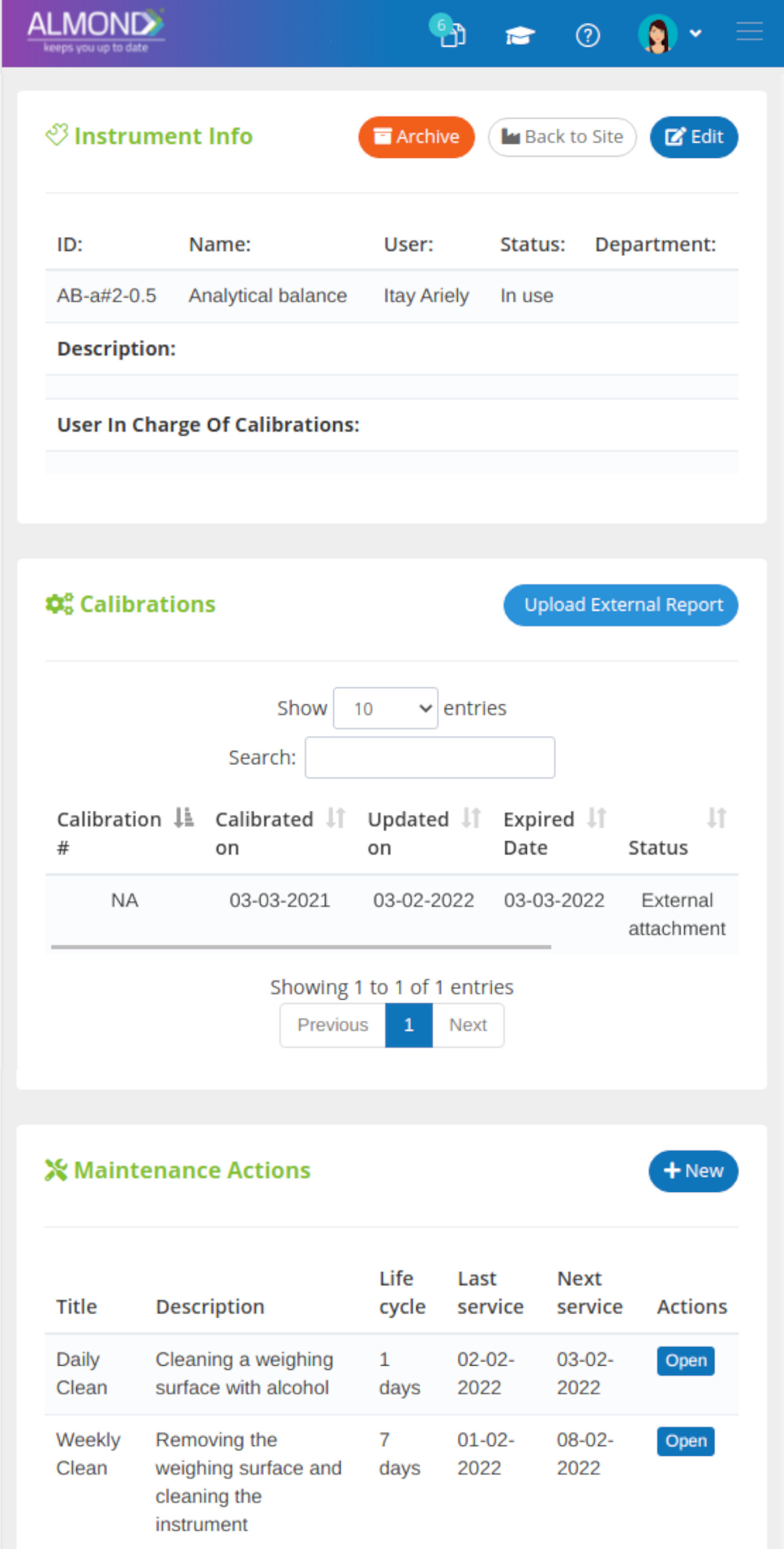
Control your equipment easily:
Smart and orderly electronic management
Create equipment groups, by categories like sites, departments, types, usage, etc.
Attach certificates and relevant documents to device file
Control calibration times and receive email notifications before calibration expiration date
Assign permission to one or more of your employees to be in charge of equipment management

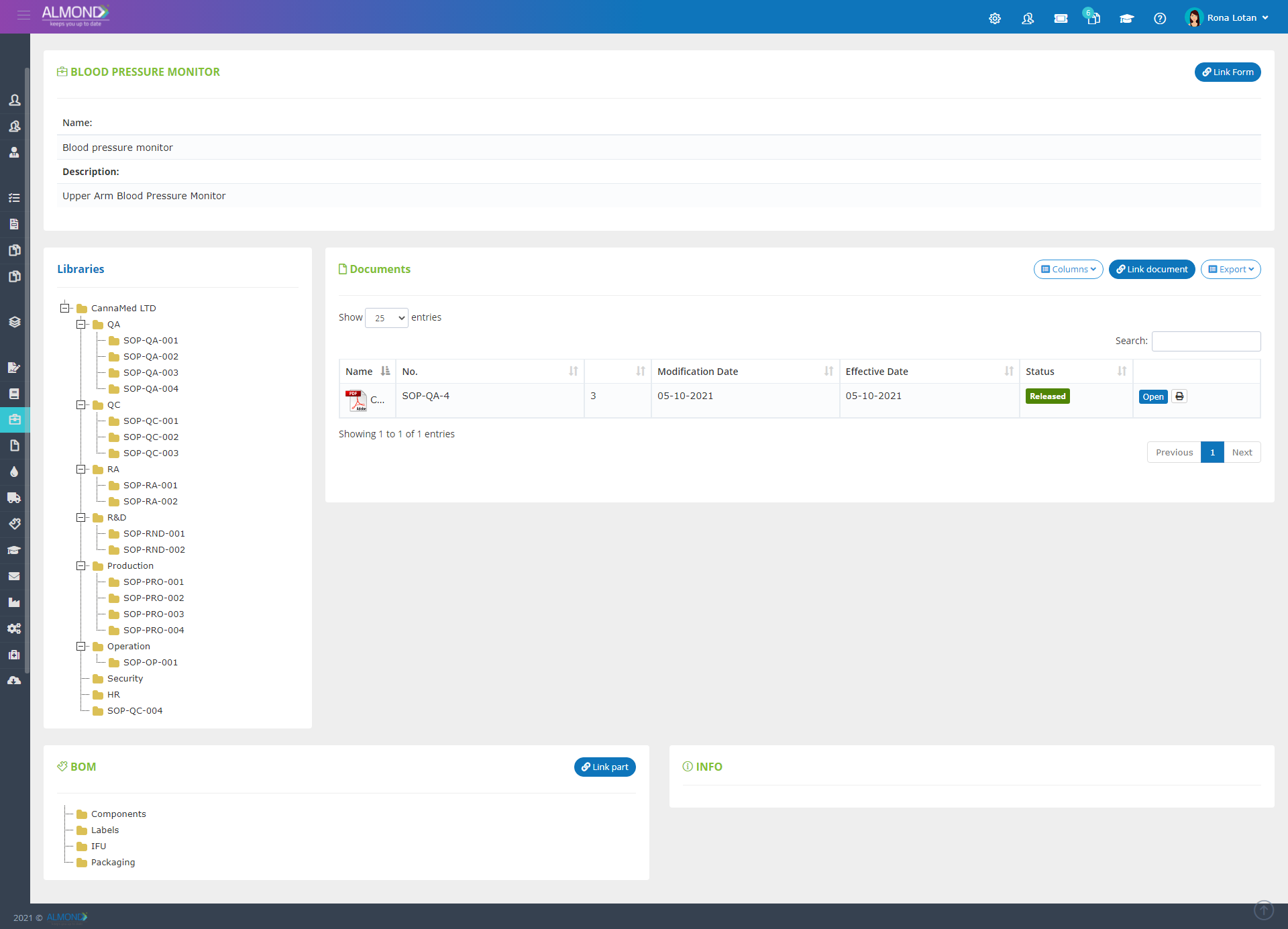
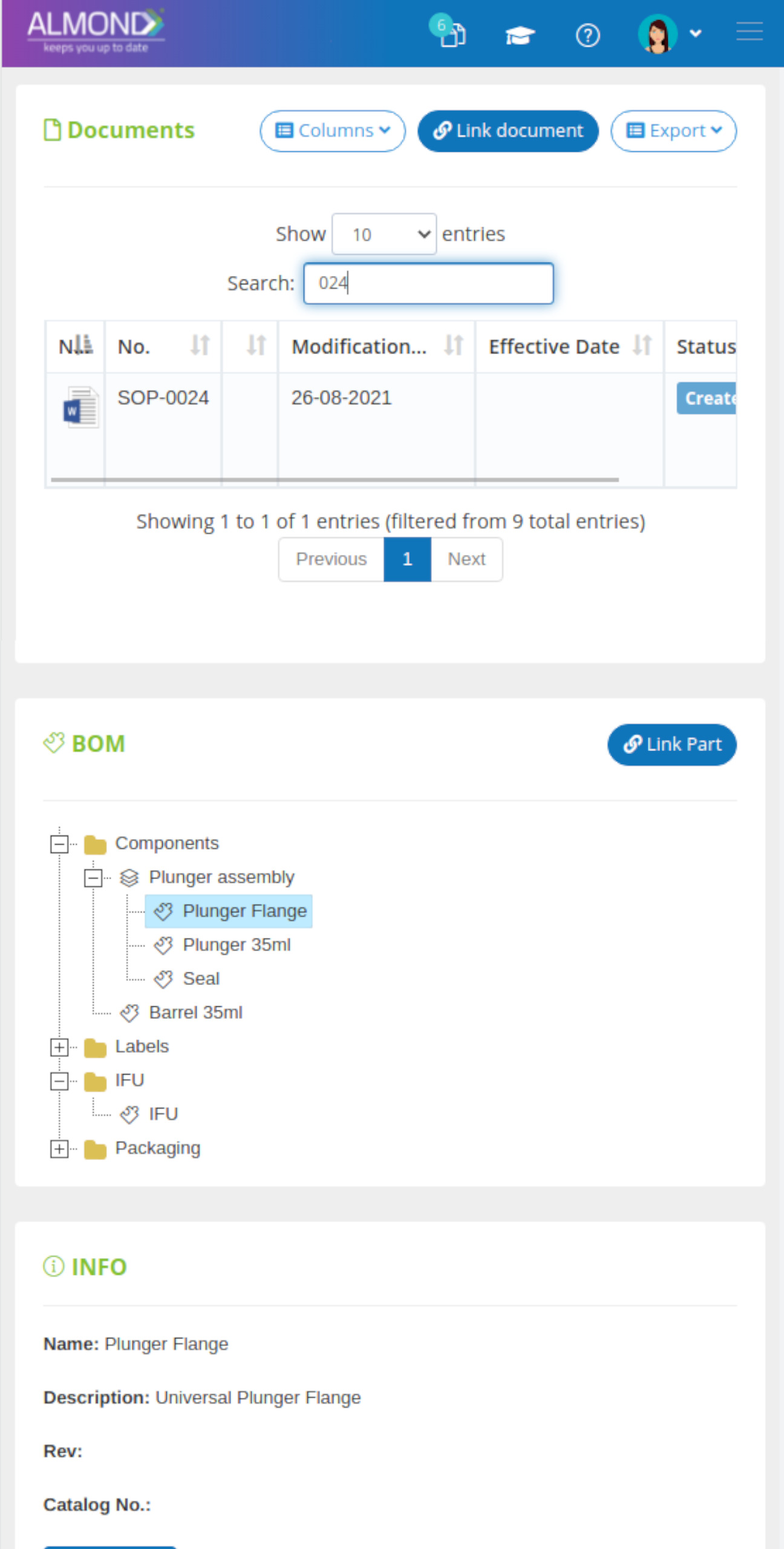
Manage your products simply and correctly:
Create easy access to all Technical documentation by linking
Documentation stage of the product design is automatically achieved when processes are performed using the system
Create and maintain batch history including automatic documentation of all records while maintaining batch and subassembly identification

- The Software
- Almond modules+
- Almond best practices+
- Industries
- Schedule a demo
Best Practices
Almond eQMS comes includes the Gsap best practices modules,
adapted to the relevant industry (pharmaceutical, medical devices, medical cannabis, etc) as follows:
adapted to the relevant industry (pharmaceutical, medical devices, medical cannabis, etc) as follows:
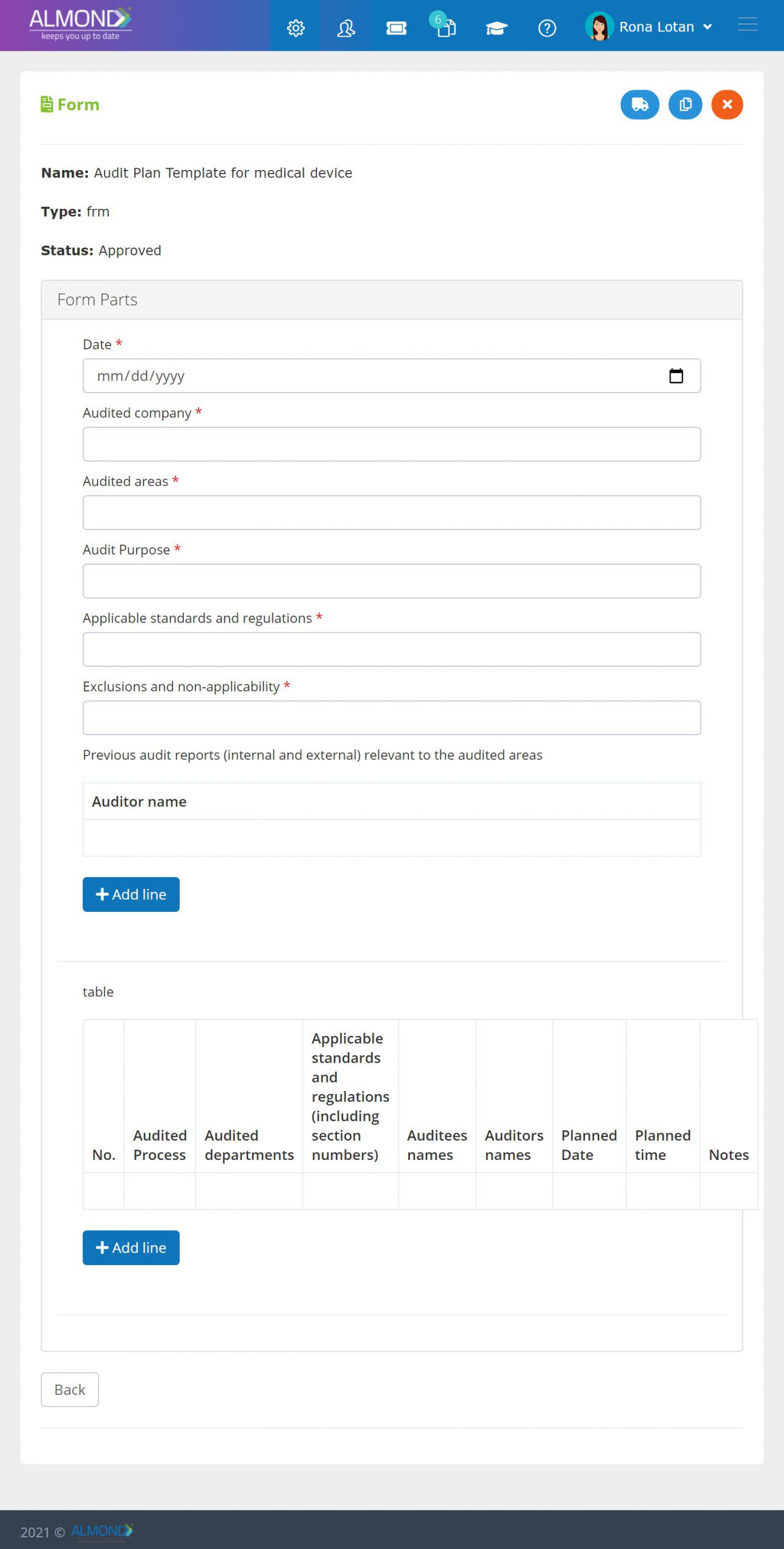
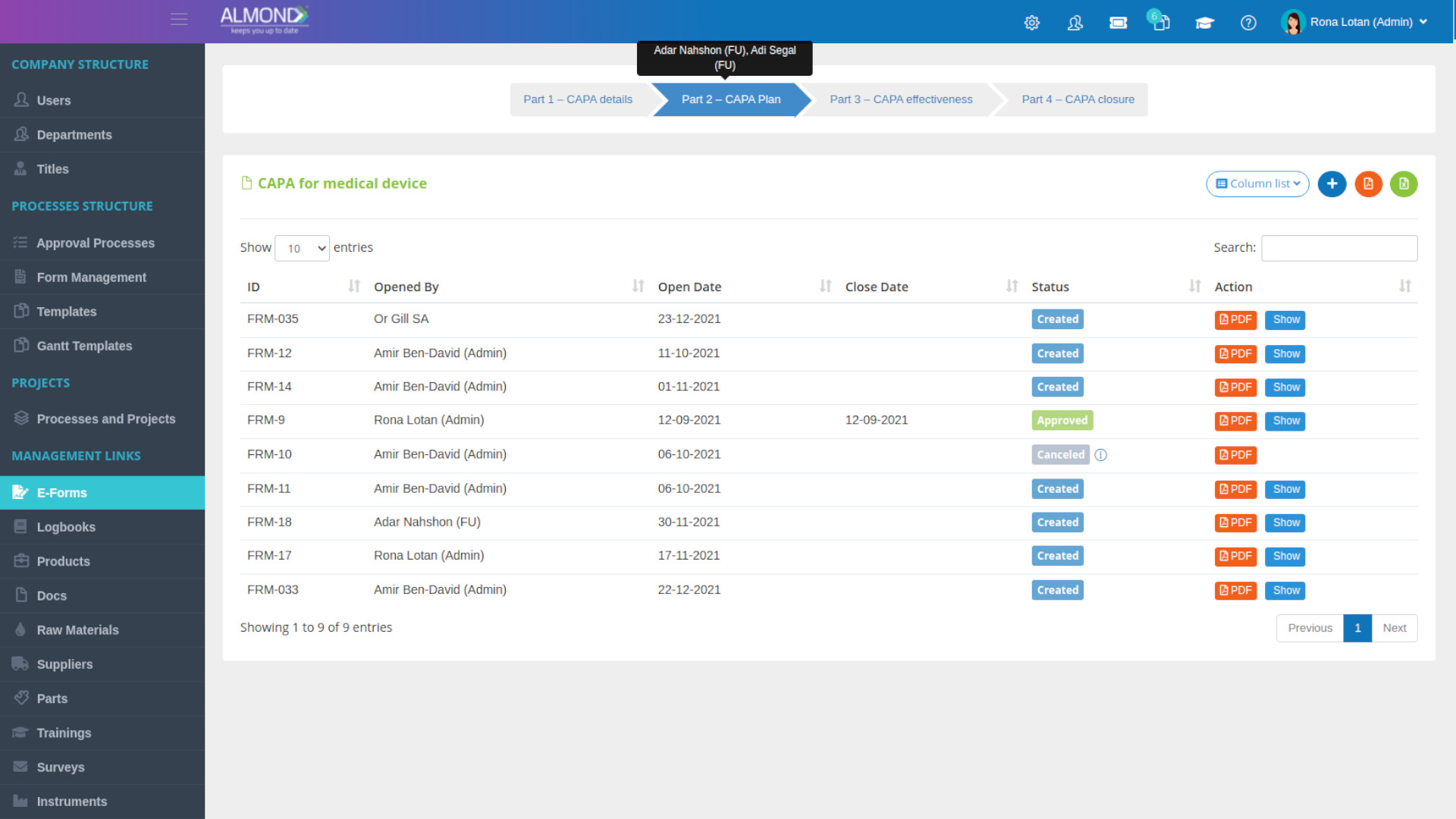
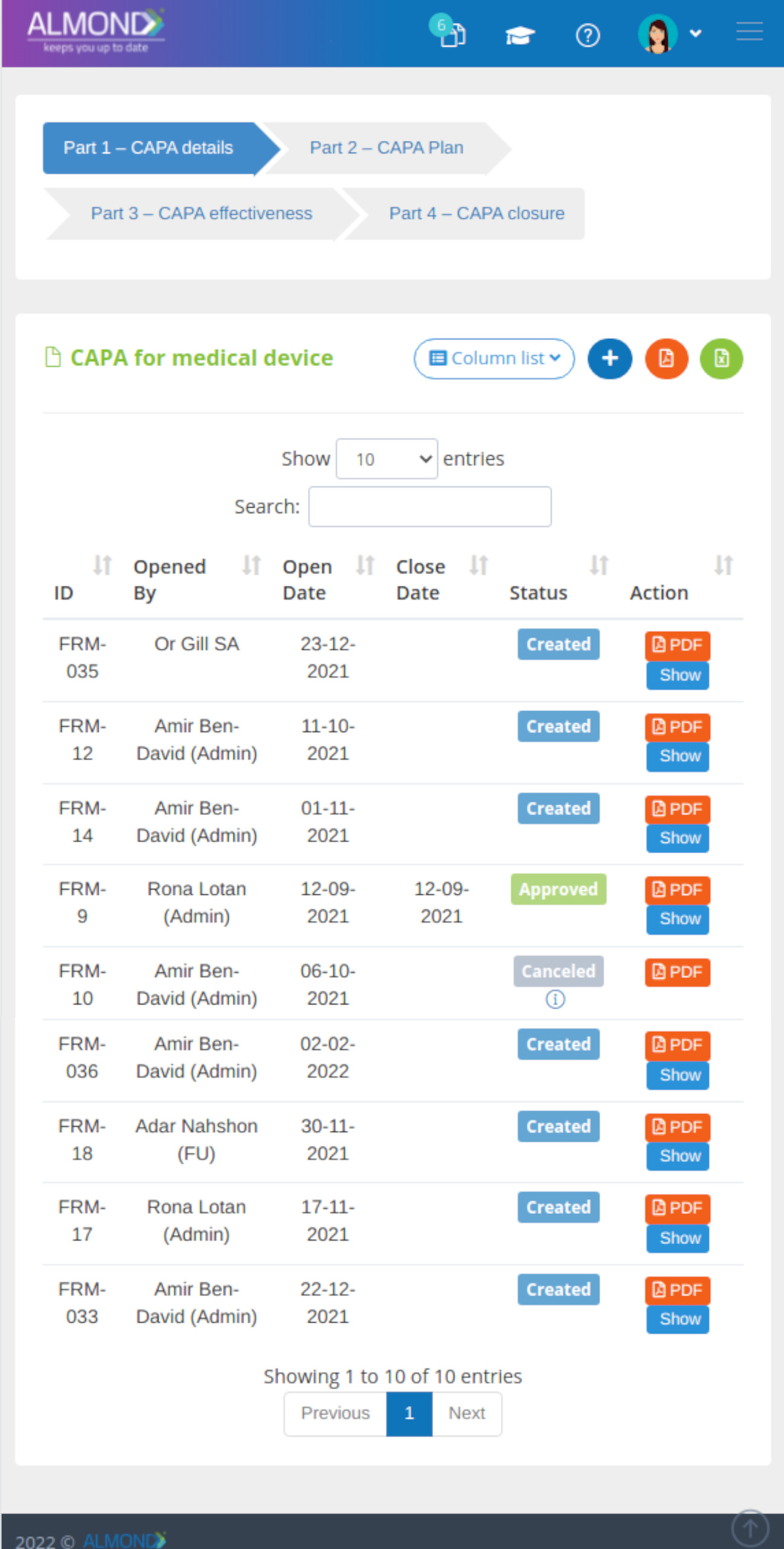
- Quality audit is the process of systematic examination of a quality system carried out by an internal or external quality auditor or an audit team.
- Almond eQMS enables capturing periodic audit plans and recording audit execution and its life cycle including managing its outcomes, such as CAPA.
- Corrective And Preventive Action, a methodological strategy for mitigating risks and improving processes by identifying sources of actual or potential issues and its root causes planning and documenting solutions to eliminate their occurrences.
- Almond eQMS enables managing the CAPA process electronically including linkage to CAPA source and its tasks.
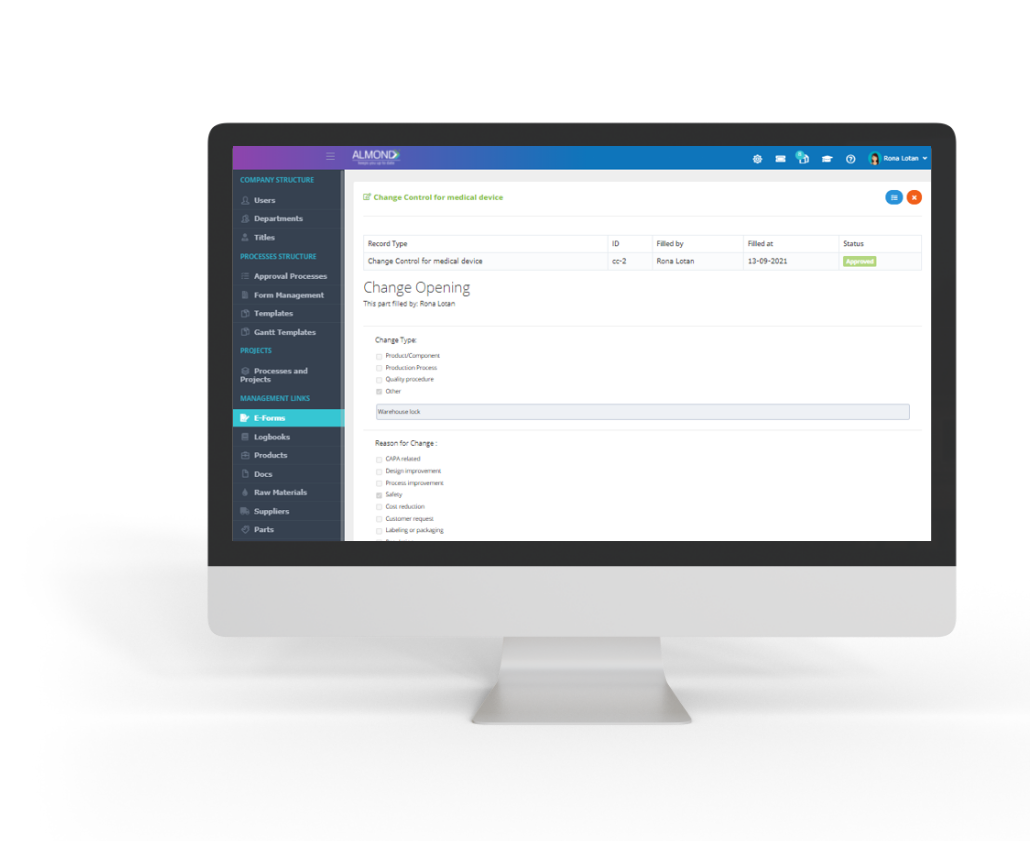
- Change control process ensures that changes to a product or system are introduced in a controlled and coordinated manner minimal but yet overlapped disruption to services
- The process allow reduction in back-out activities, and cost-effective utilization of resources involved in implementing change.
- Almond eQMS enables to record the process flow as well as capturing all applicable linkages.
- Change request is preliminary phase of change control management process.
- change request states needs to be accomplished, but leaves out how the change should be carried out.
- Almond eQMS enables to record the process flow as well as capturing all applicable linkages.
- Customer complaint in accordance to FDA is any written, electronic, or oral communication that.
- alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness, or. performance of a device after it is released for distribution'.
- Almond eQMS enables managing customer complaint records by linkage to all applicable information.
- Deviations in accordance to FDA, is any unwanted event that represents a departure from approved processes or procedures or instruction or specification or established standard or from what is required.
- Deviations can occur during manufacturing, packing, sampling and testing of drug products.
- Almond eQMS enables managing deviation records by linkage to all applicable information.
- Nonconformance report is a tool to maintain and record any non-conformity found in in any process related to the product.
- Almond eQMS enables managing the nonconformity record and process.
- In addition, Almond eQMS will enable configurable alerts and dashboards to drill down across the supply chain and isolate failures as needed.