Concentrate on work. Leave the quality management to us.
A simple and efficient QMS software. From Gsap.

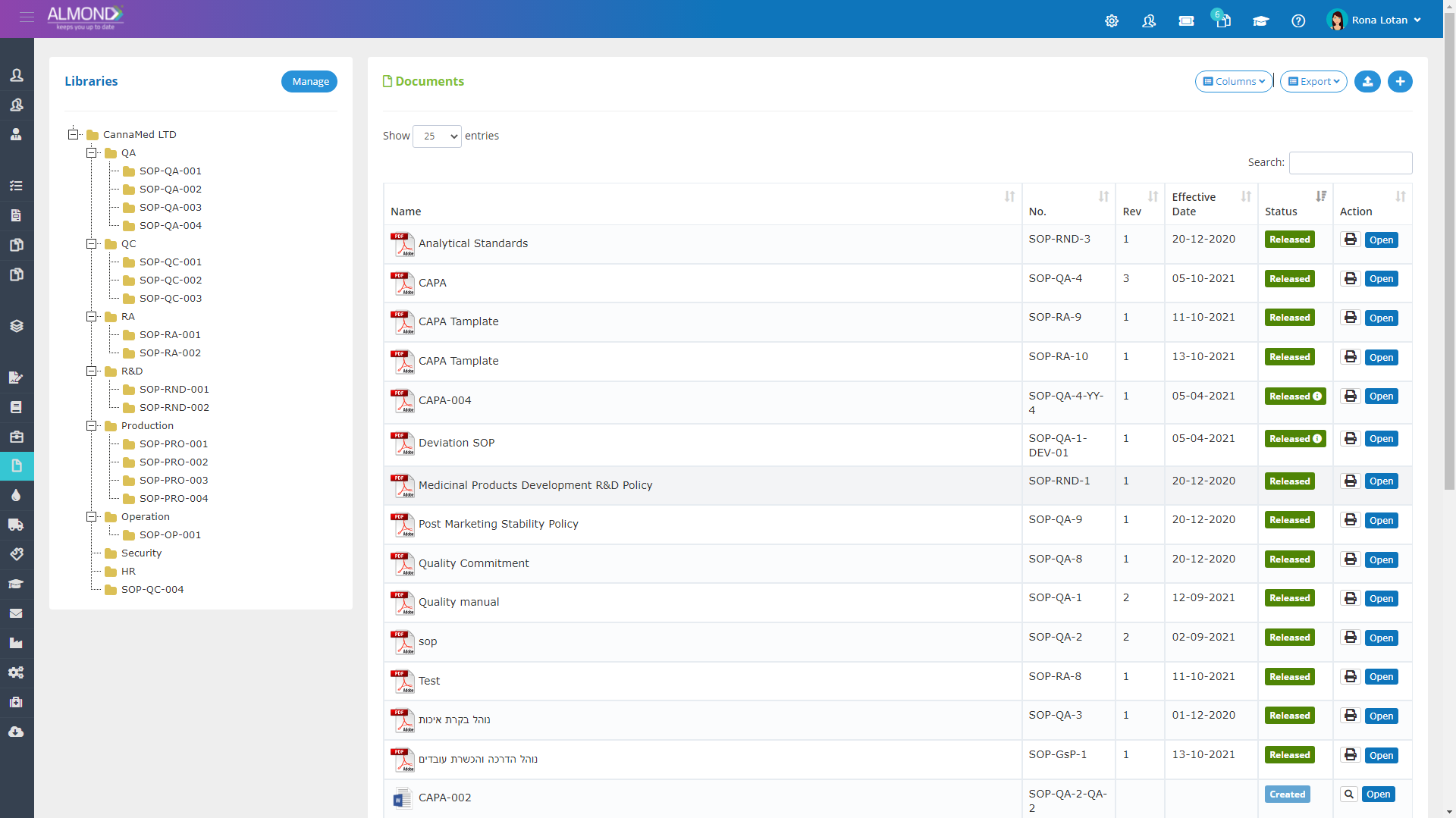
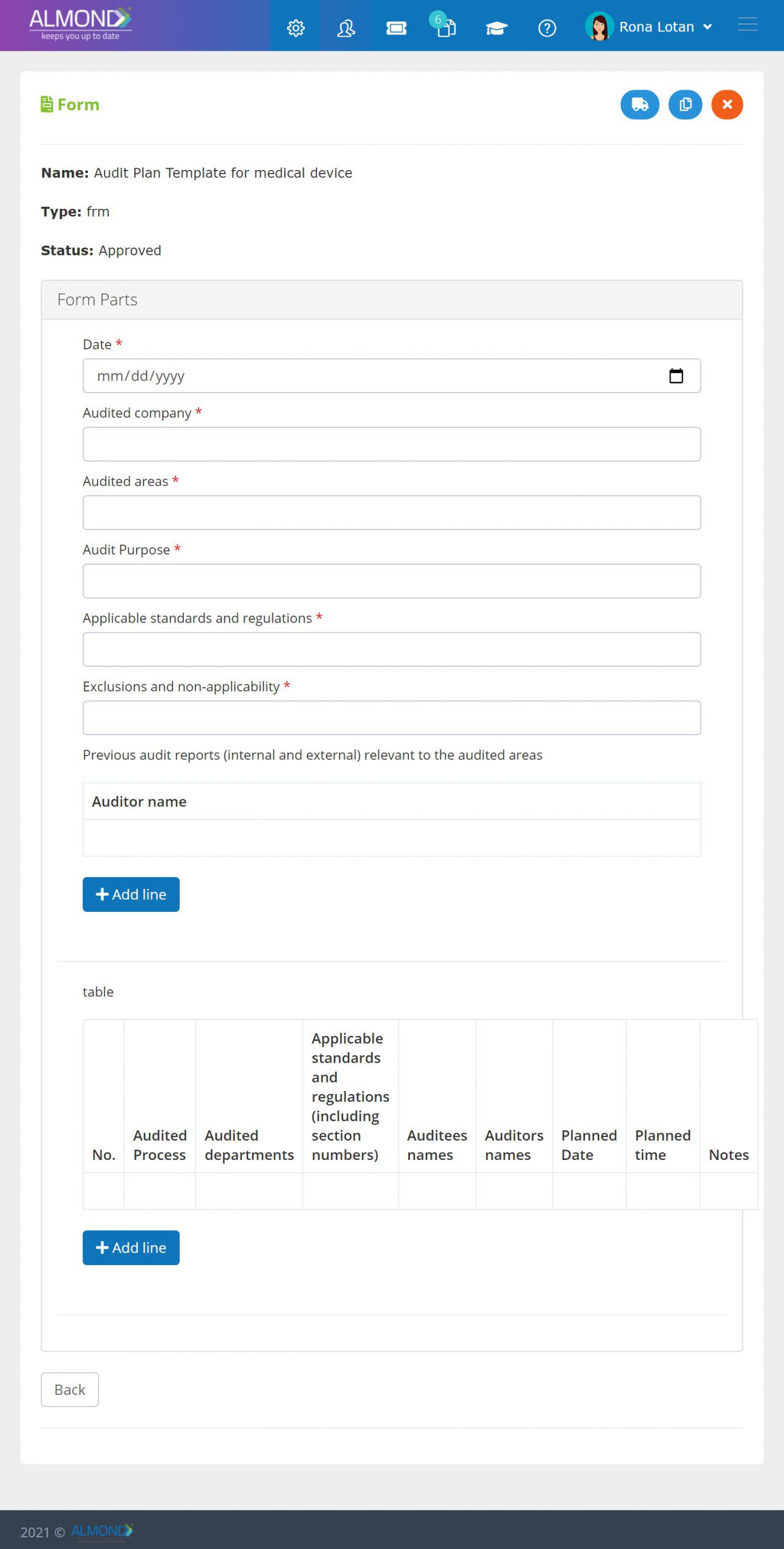
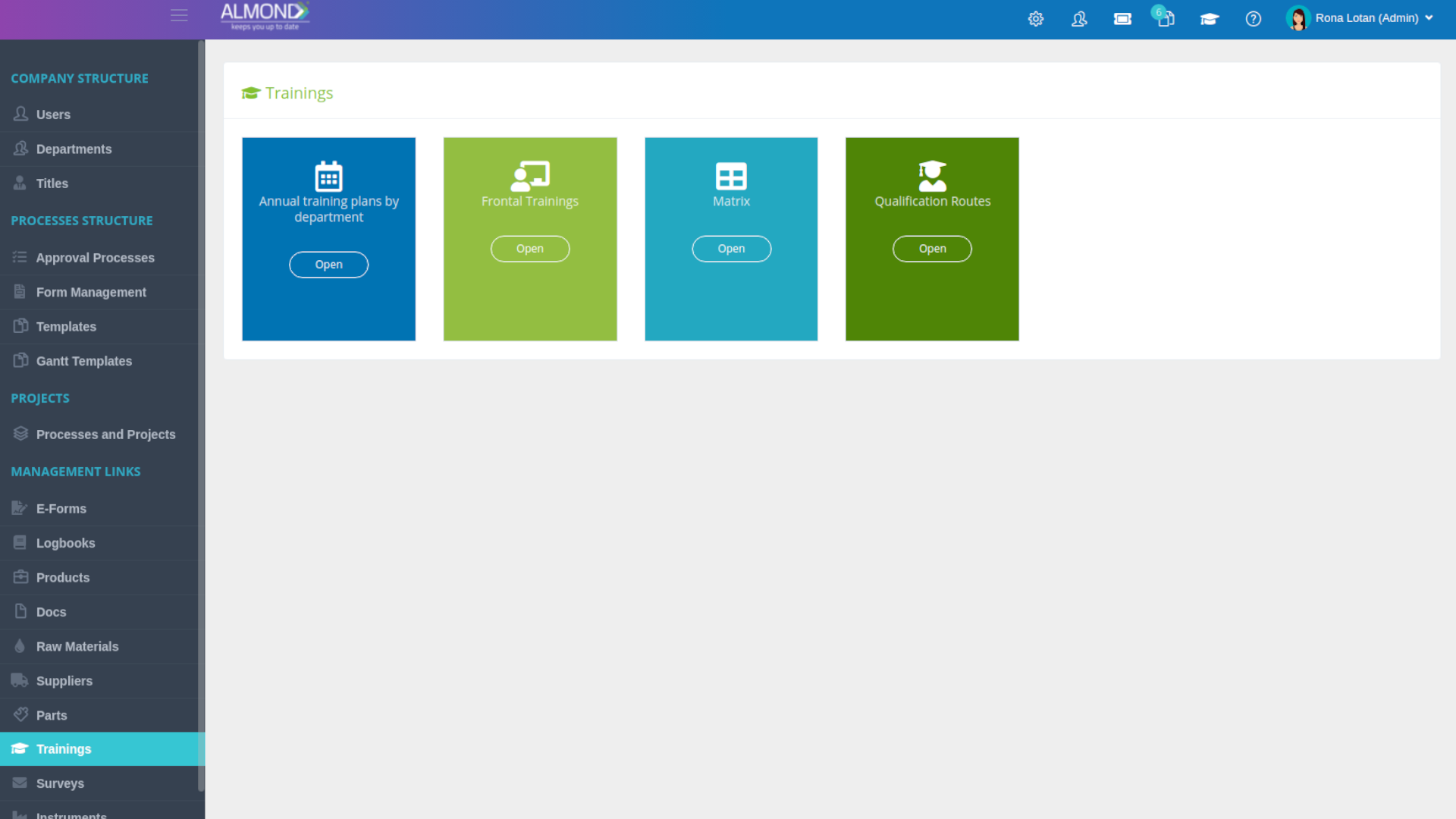
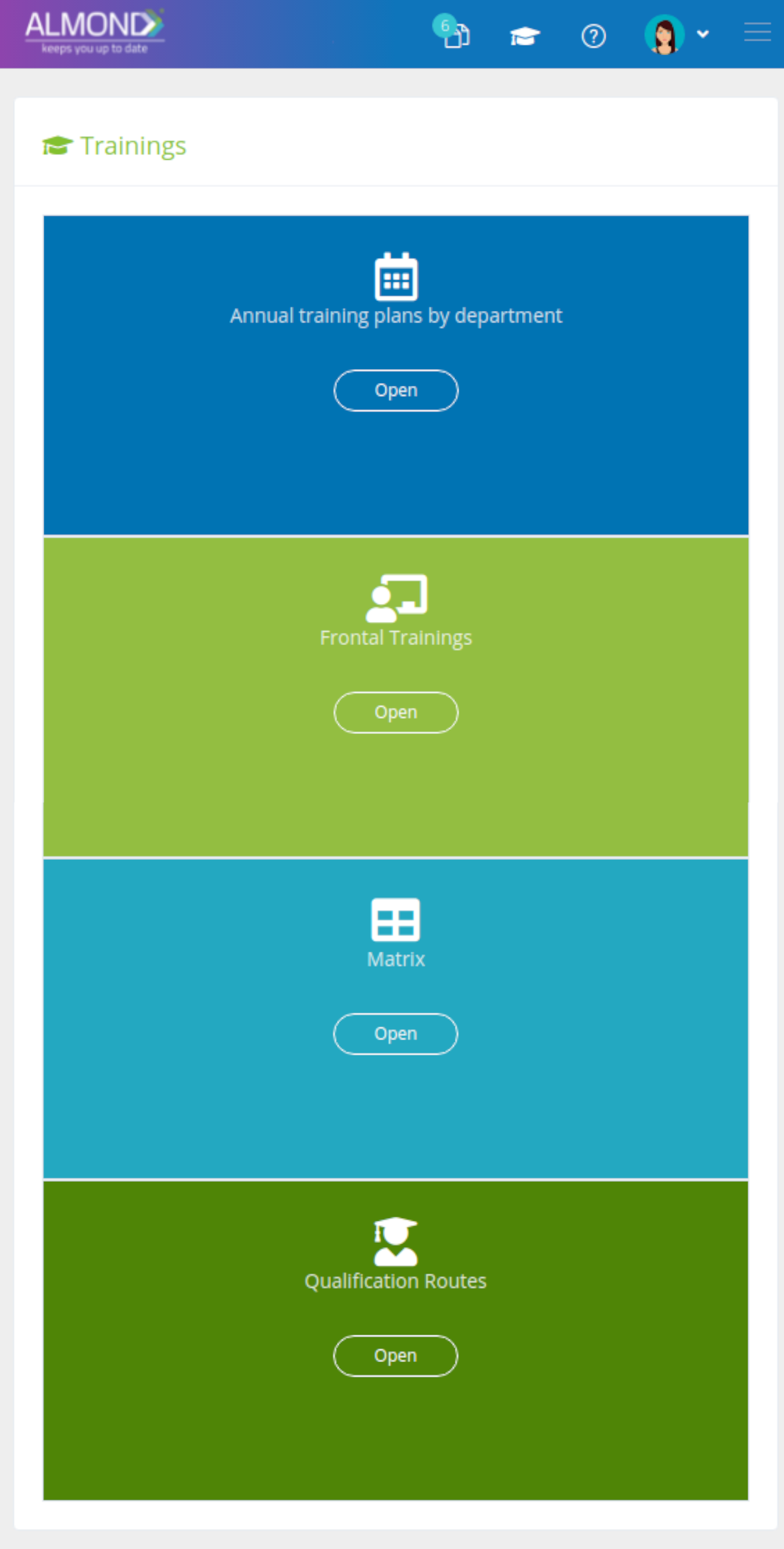
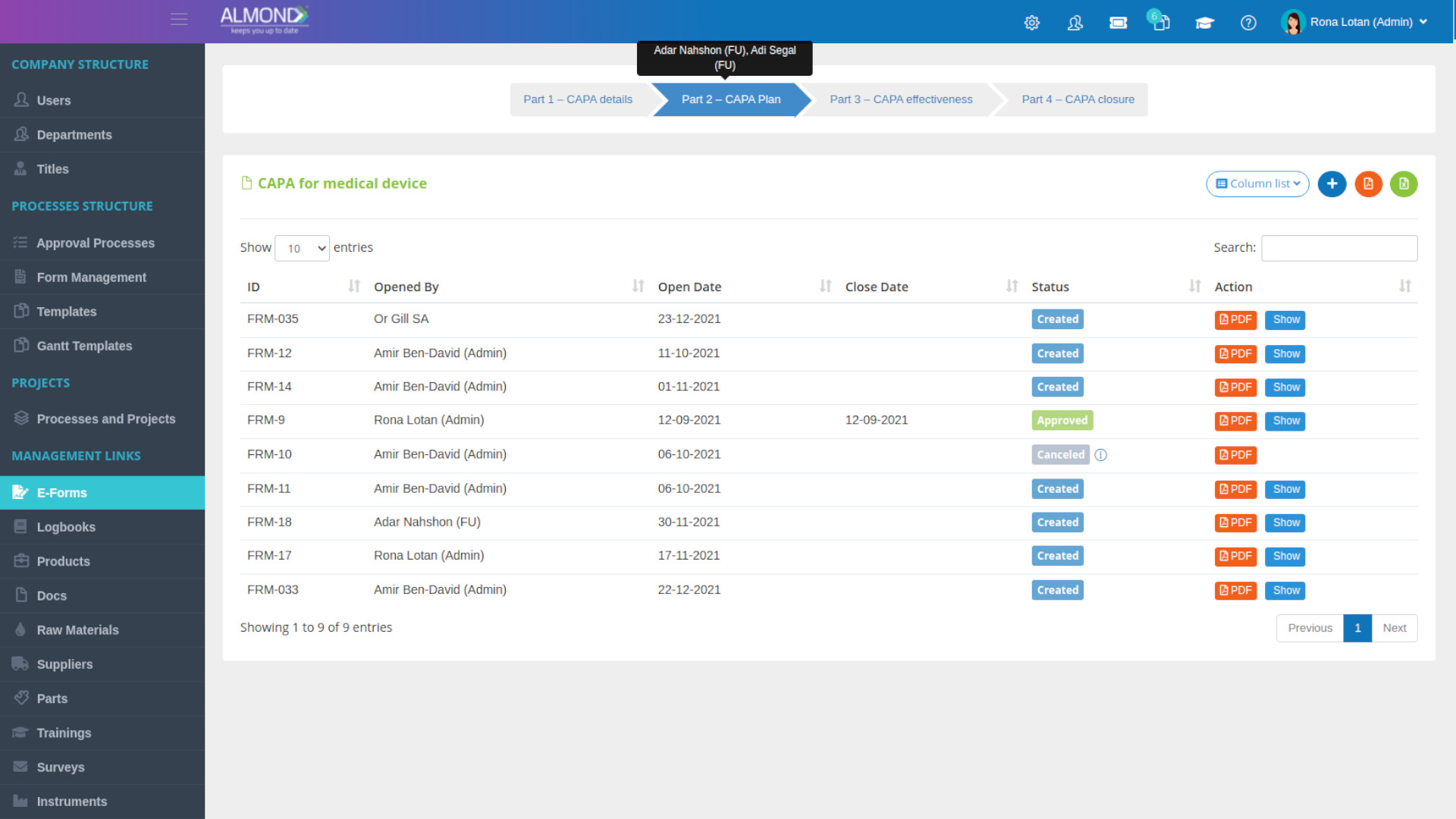
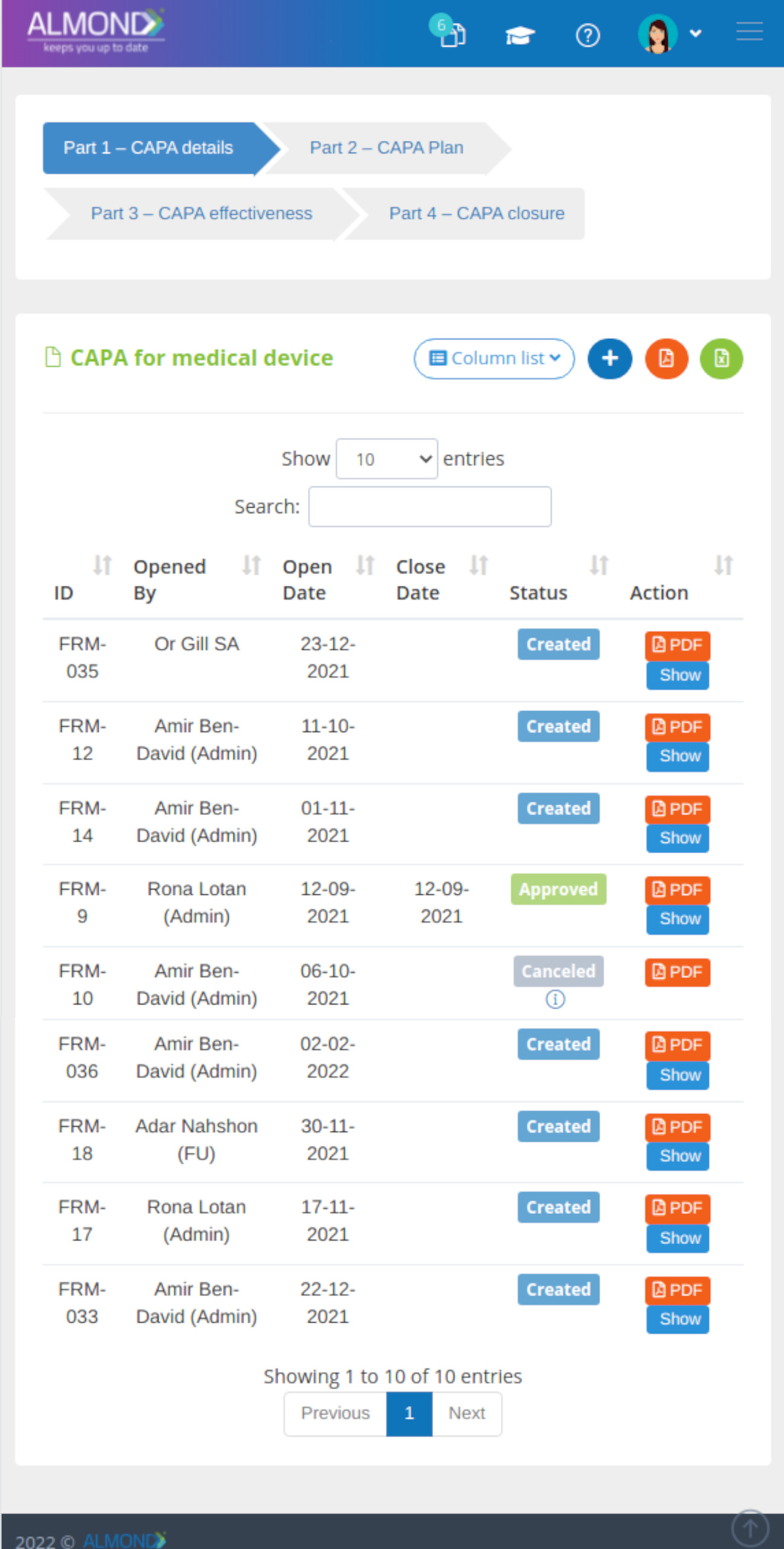
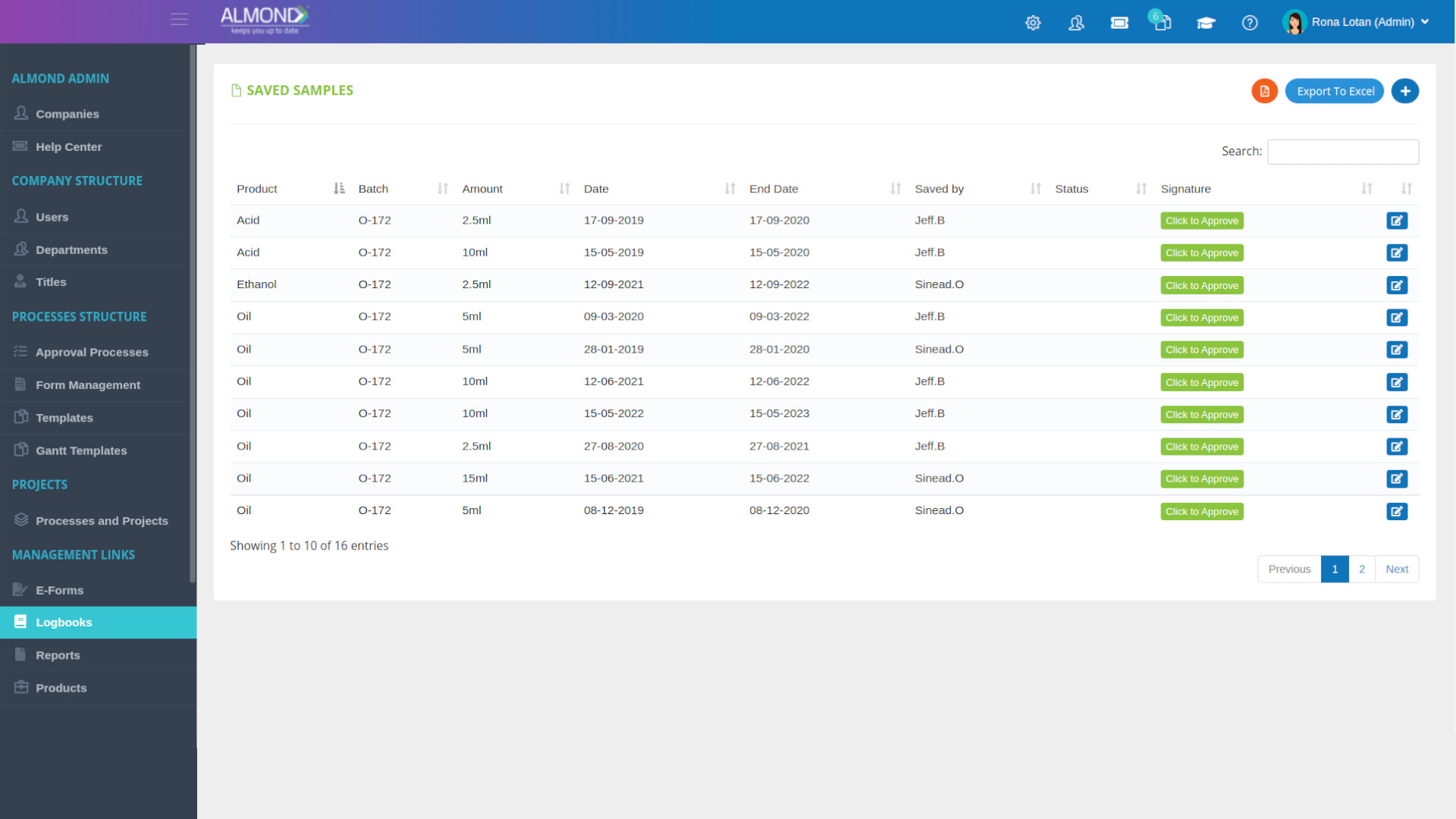
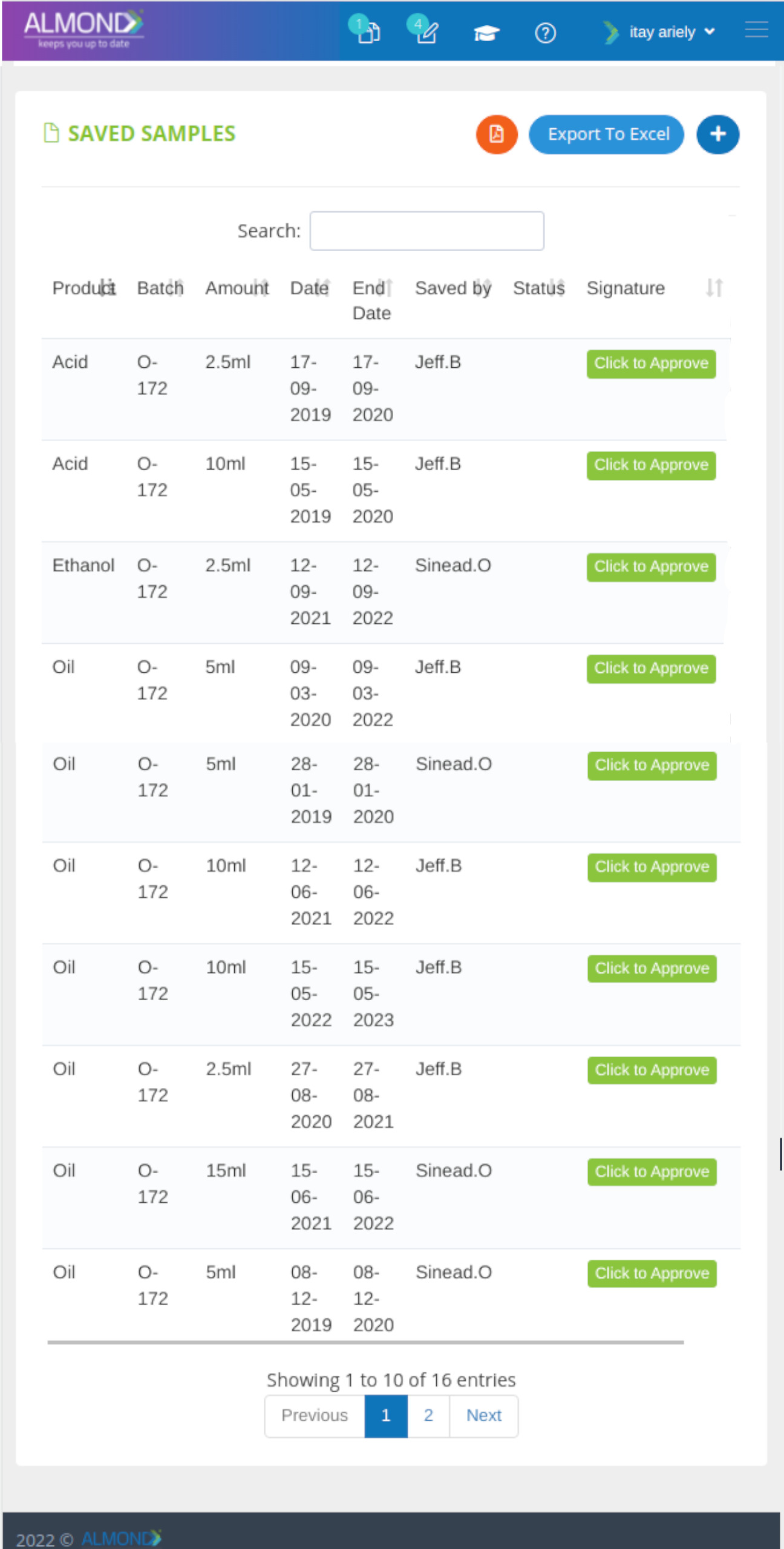
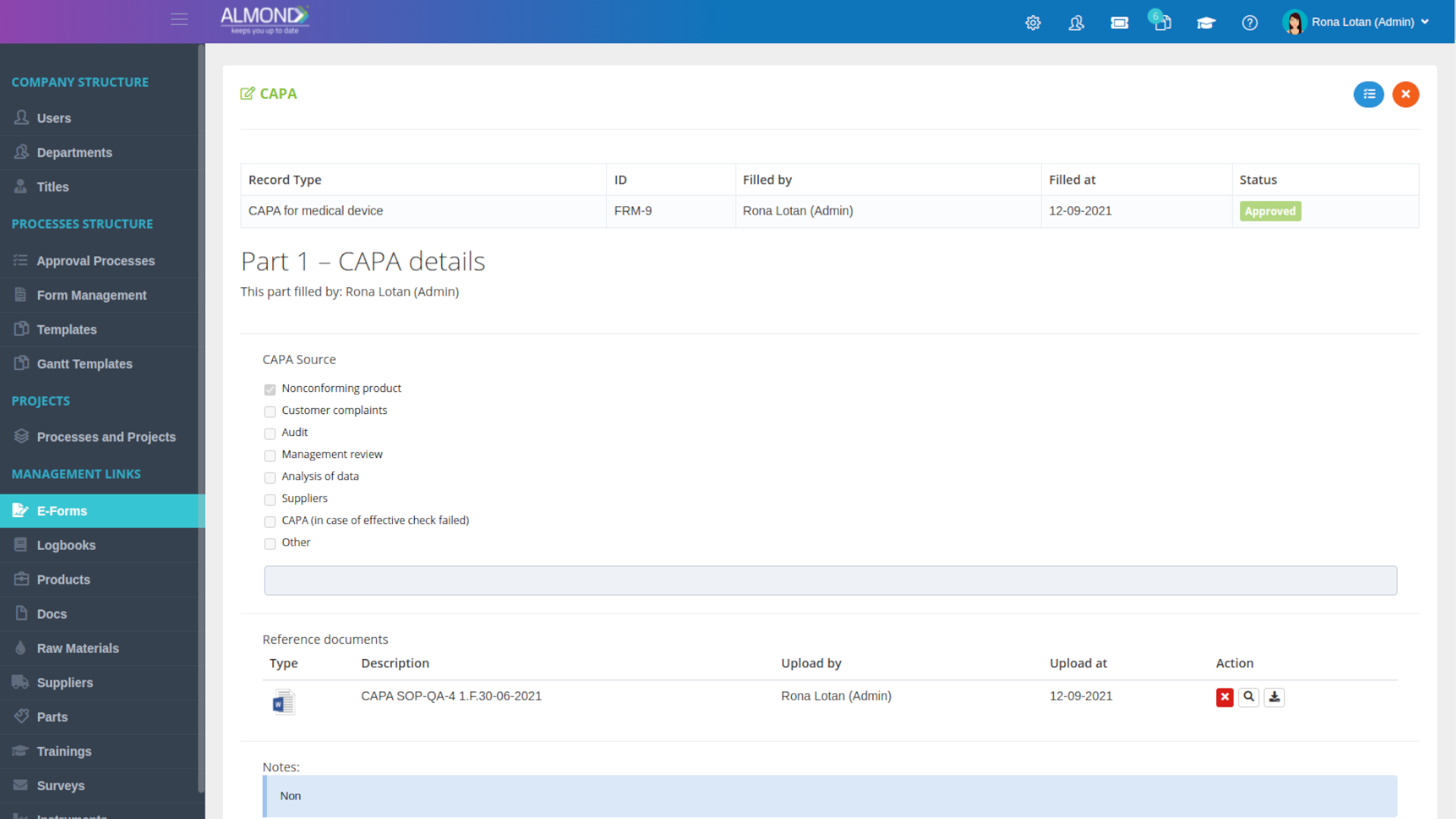
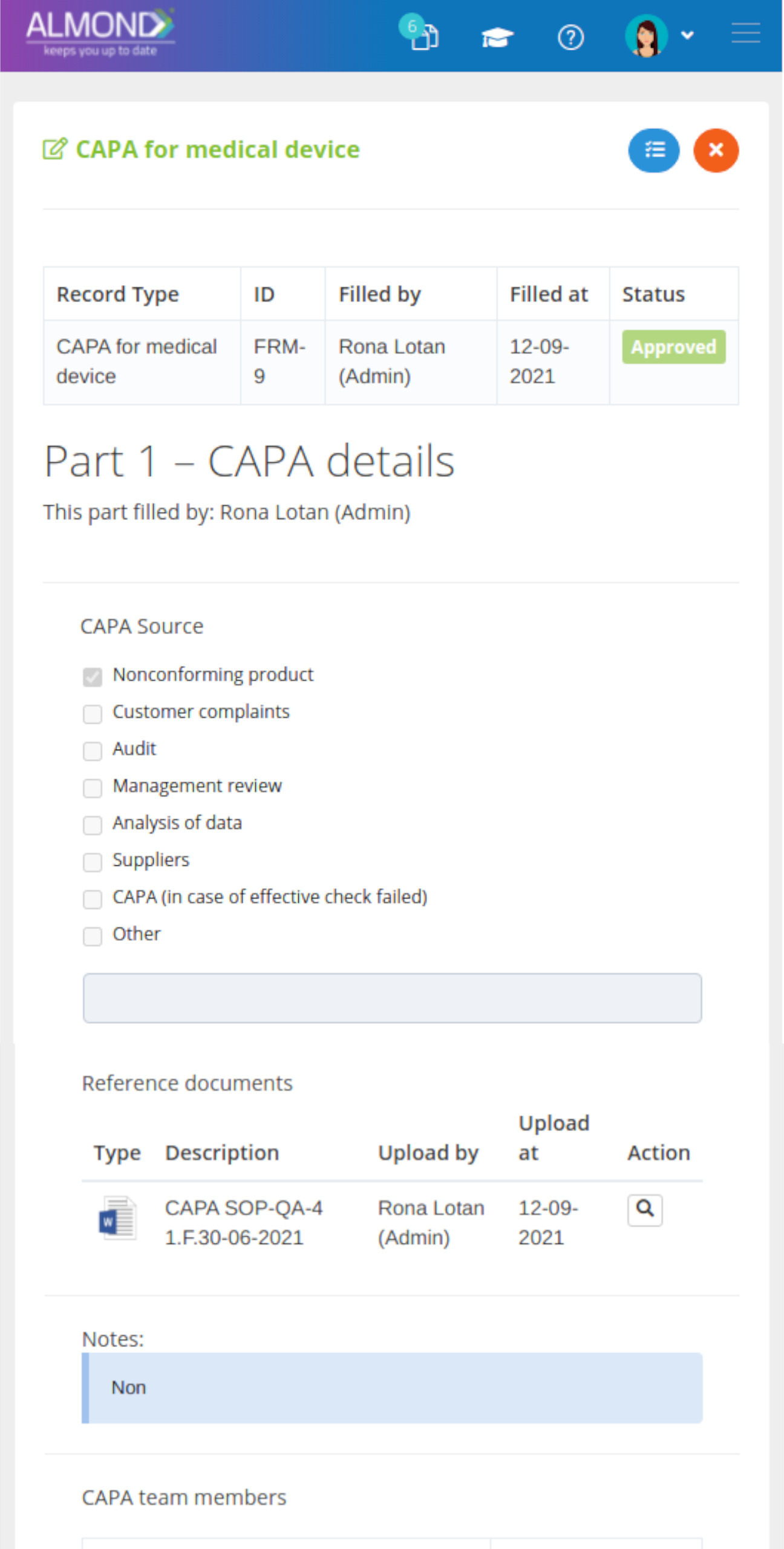
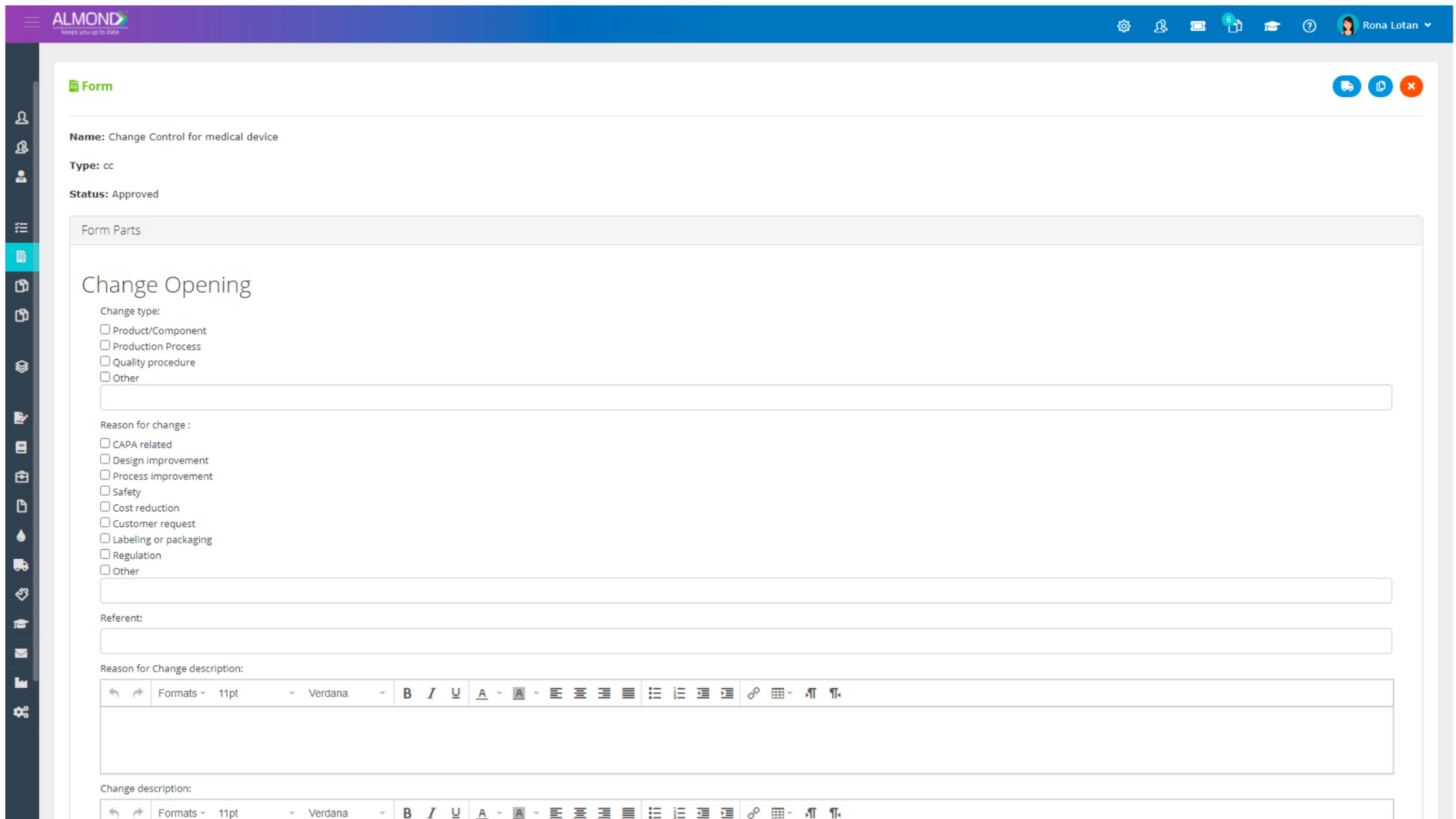
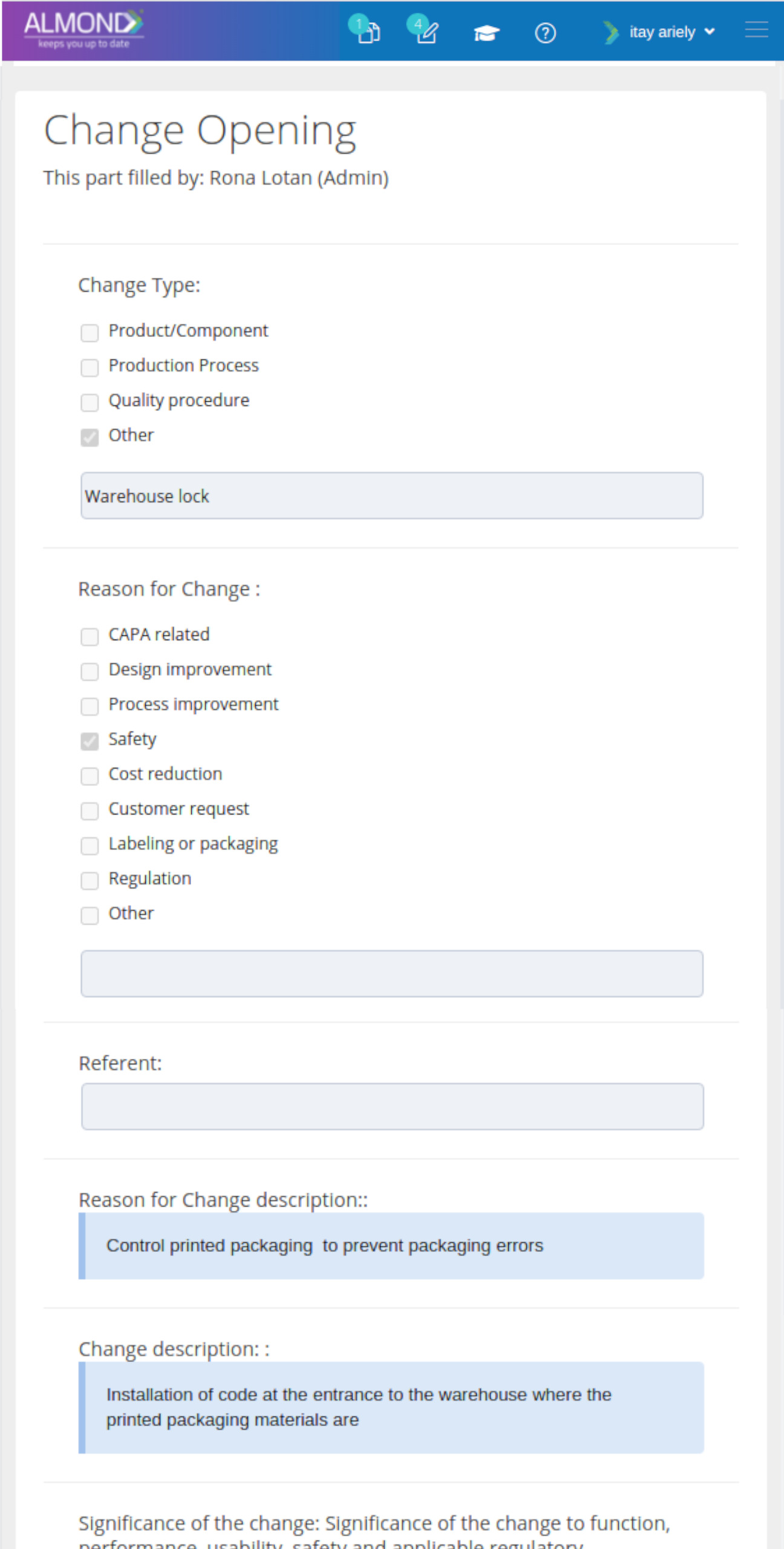
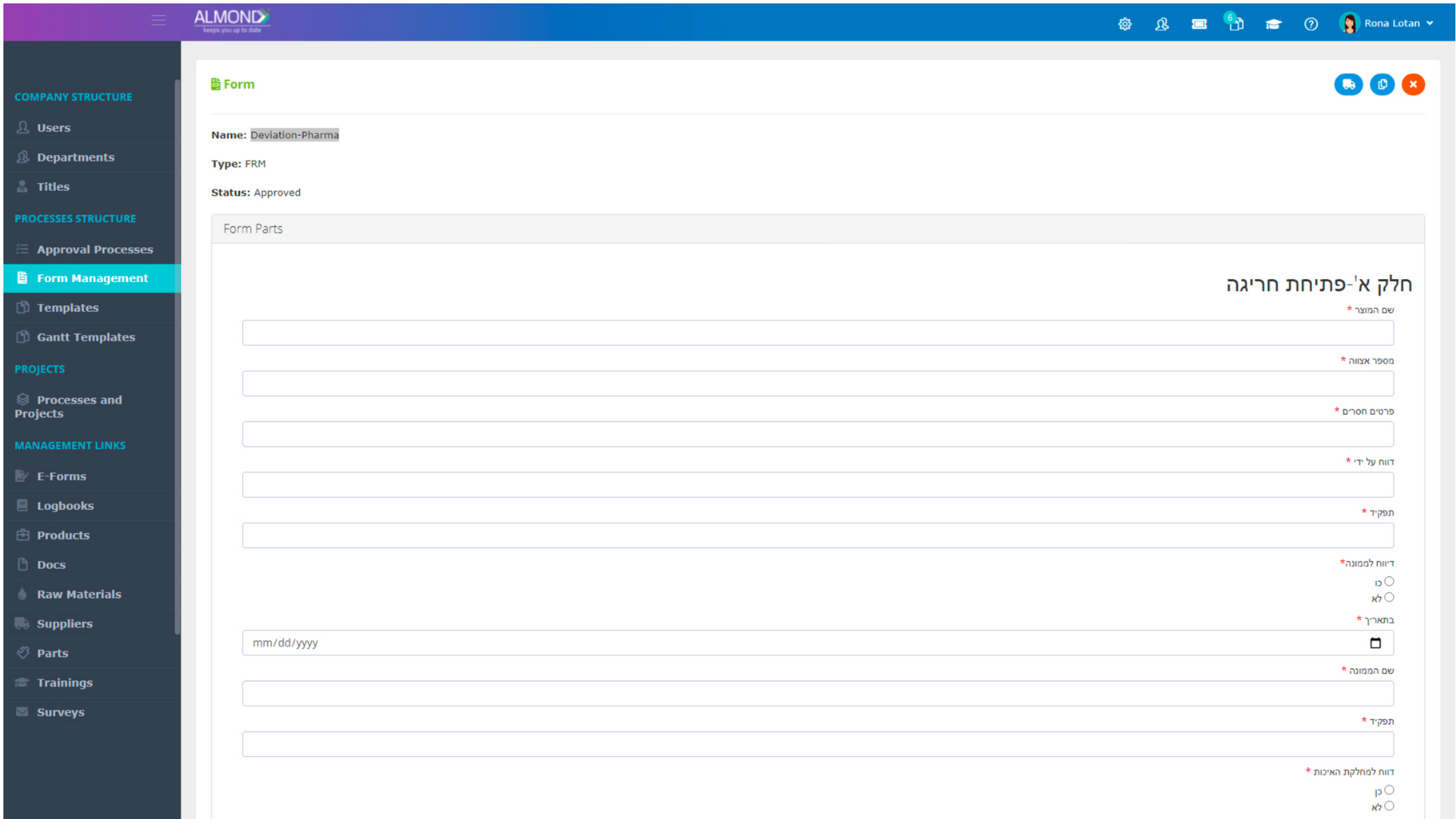
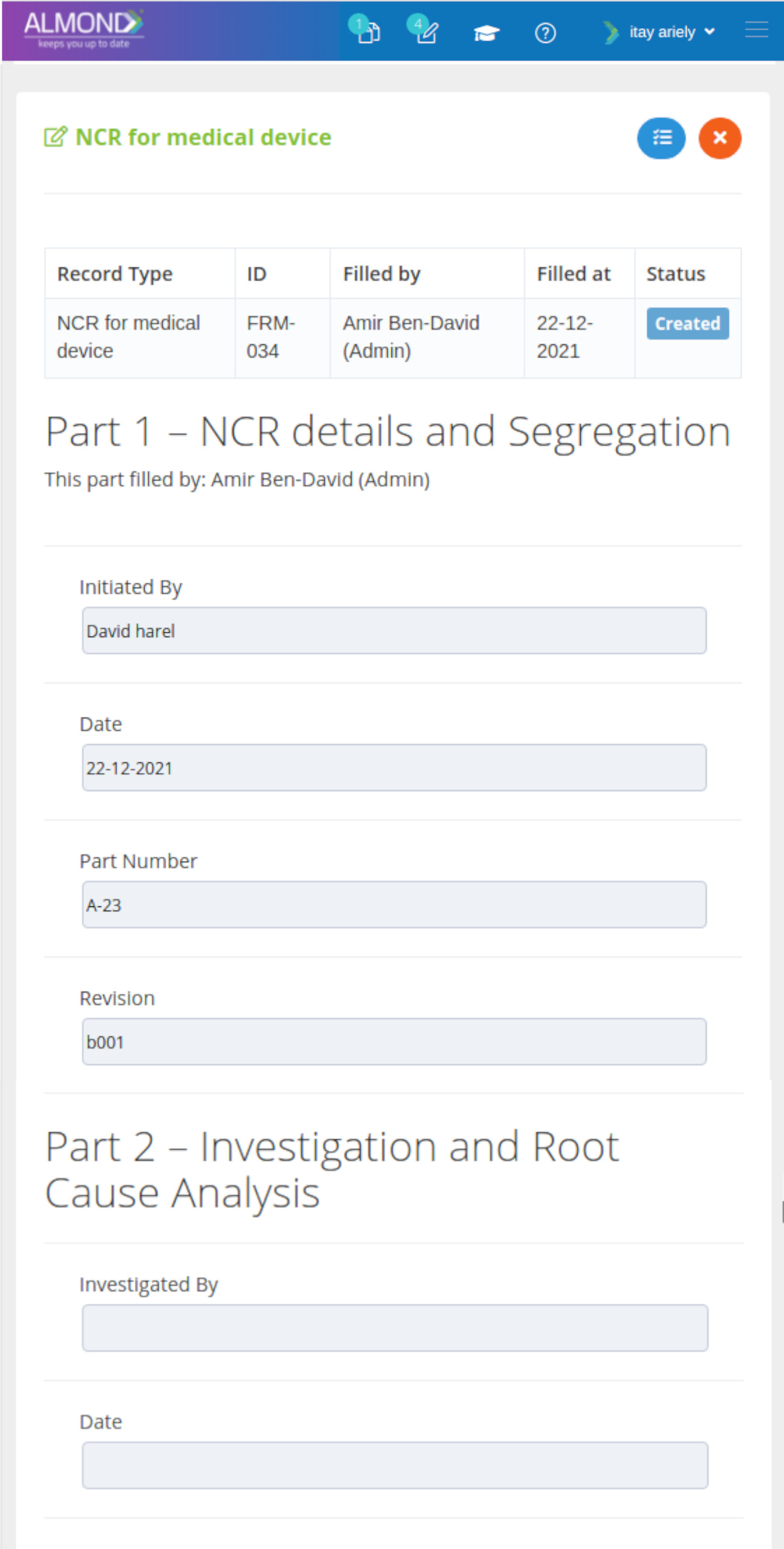
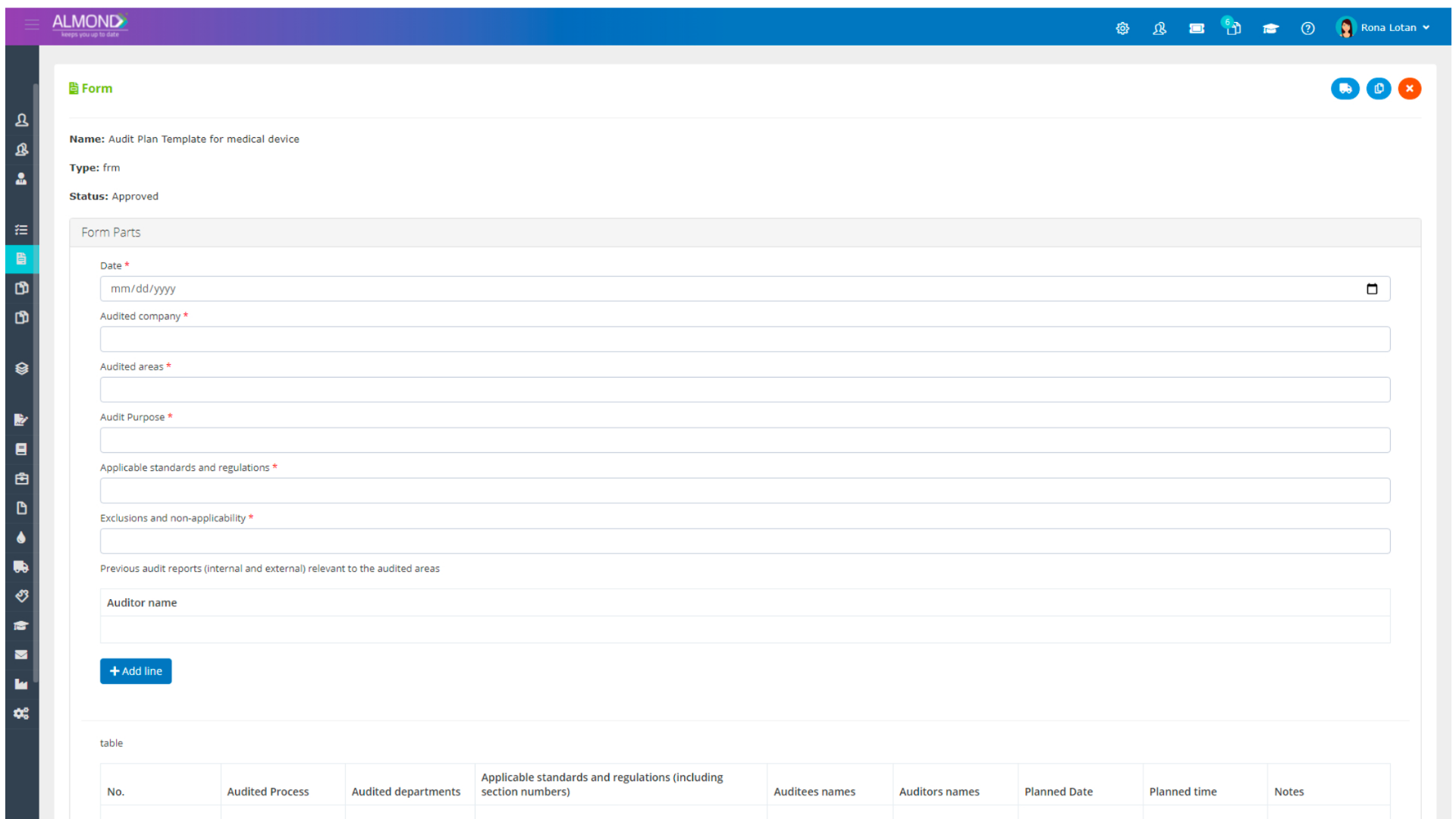
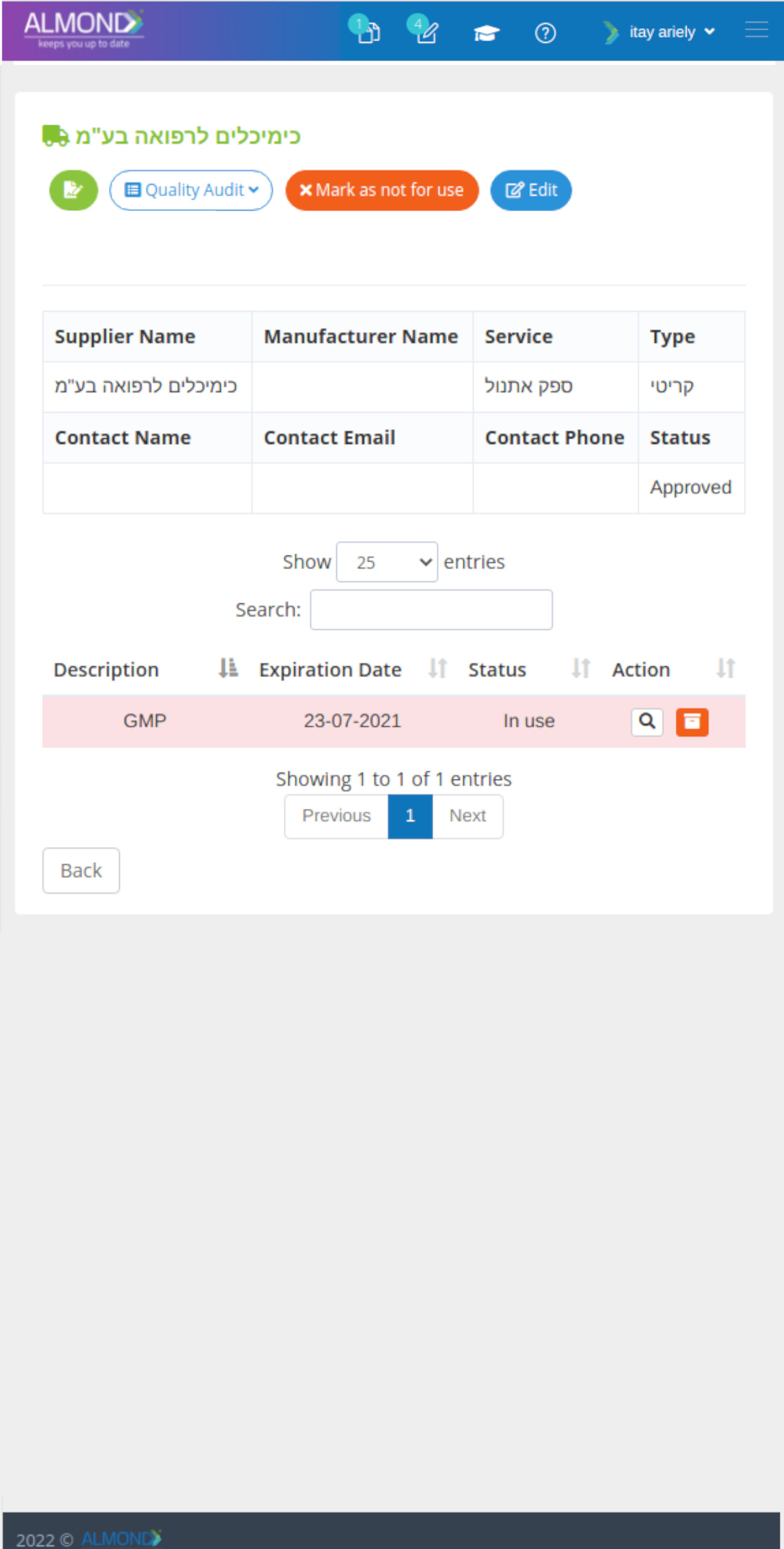
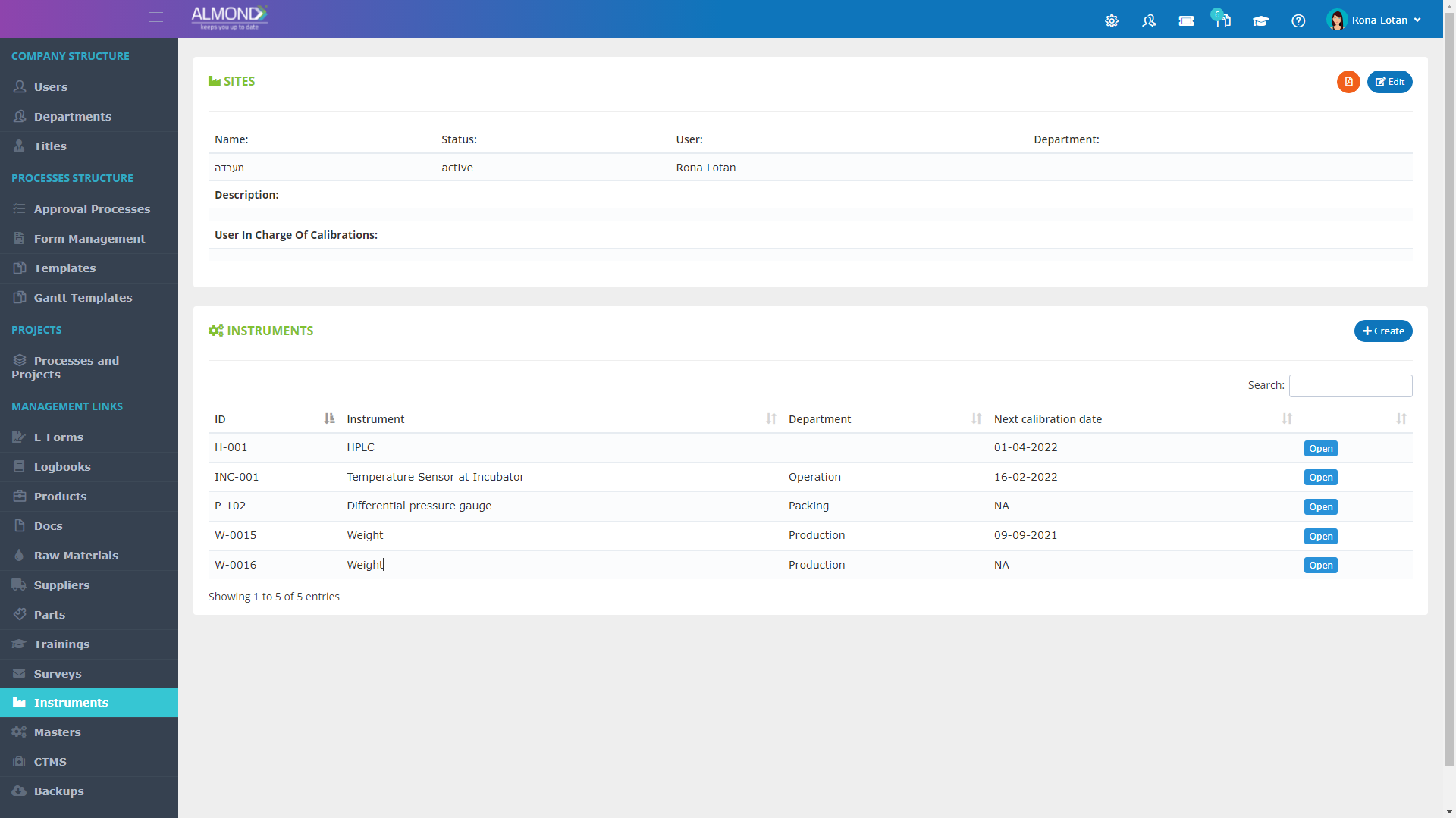
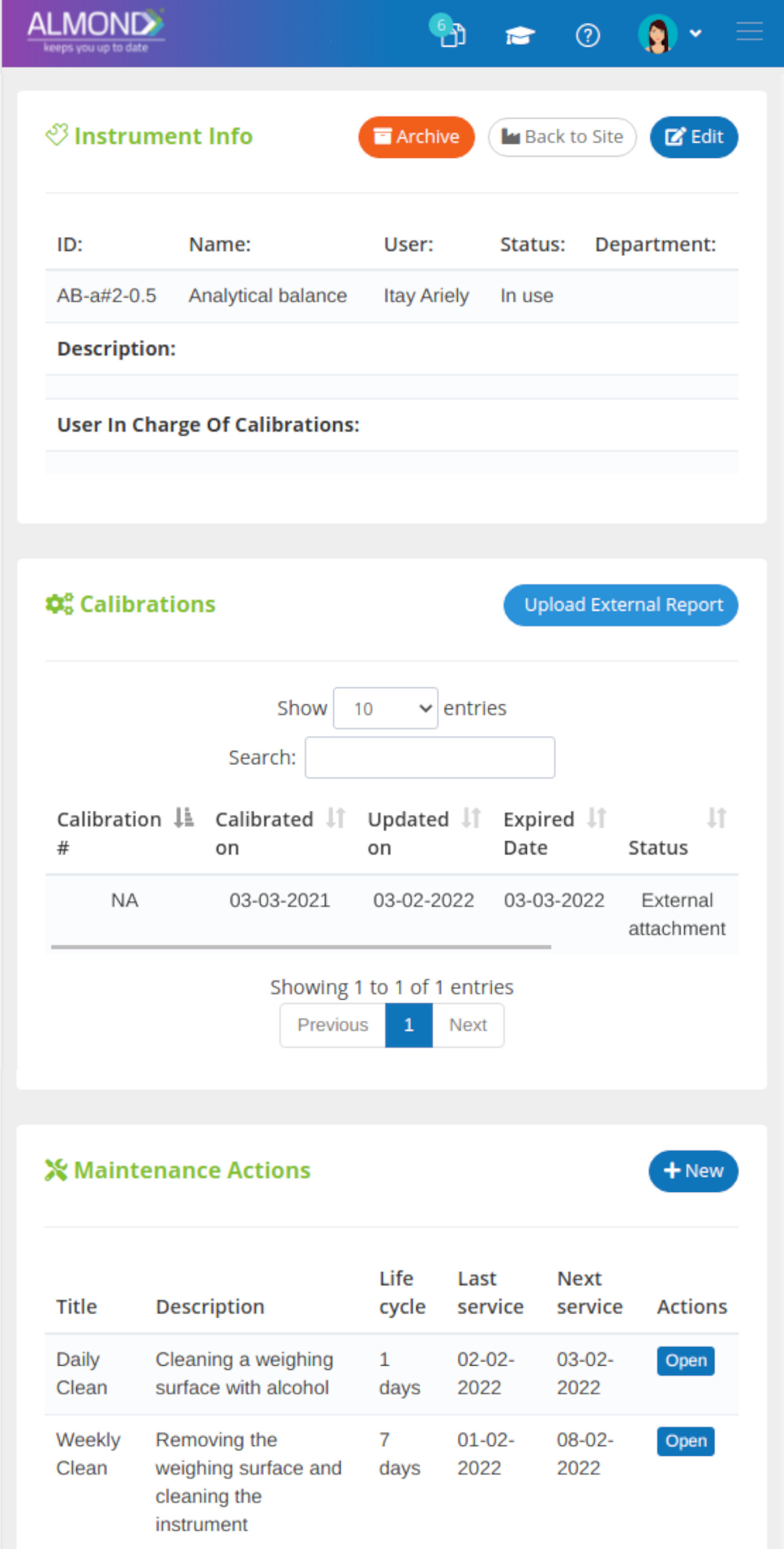
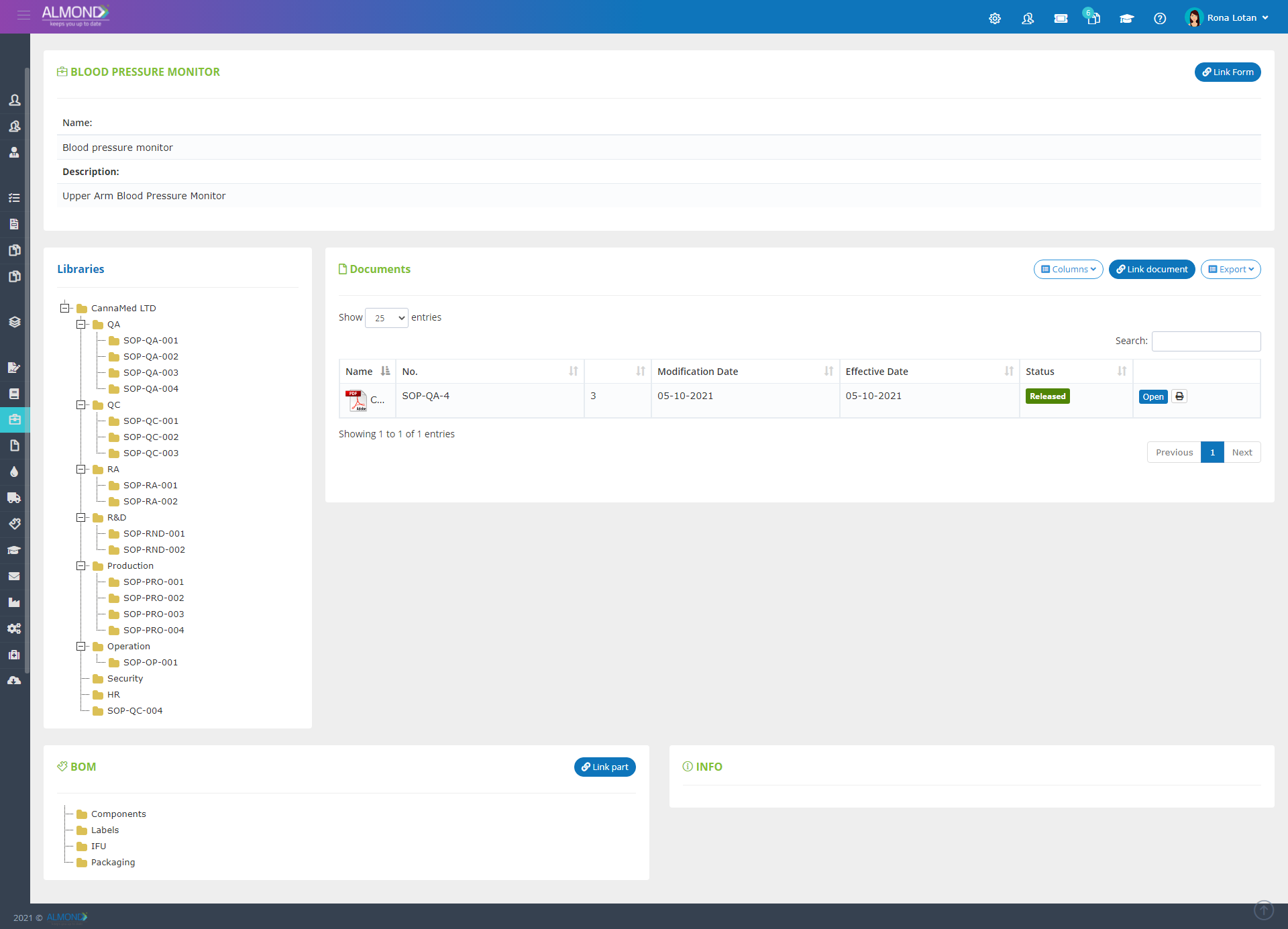
We provide an eQMS software for healthcare companies
Smooth, simple and user-friendly, ensuring regulatory compliance in every stage
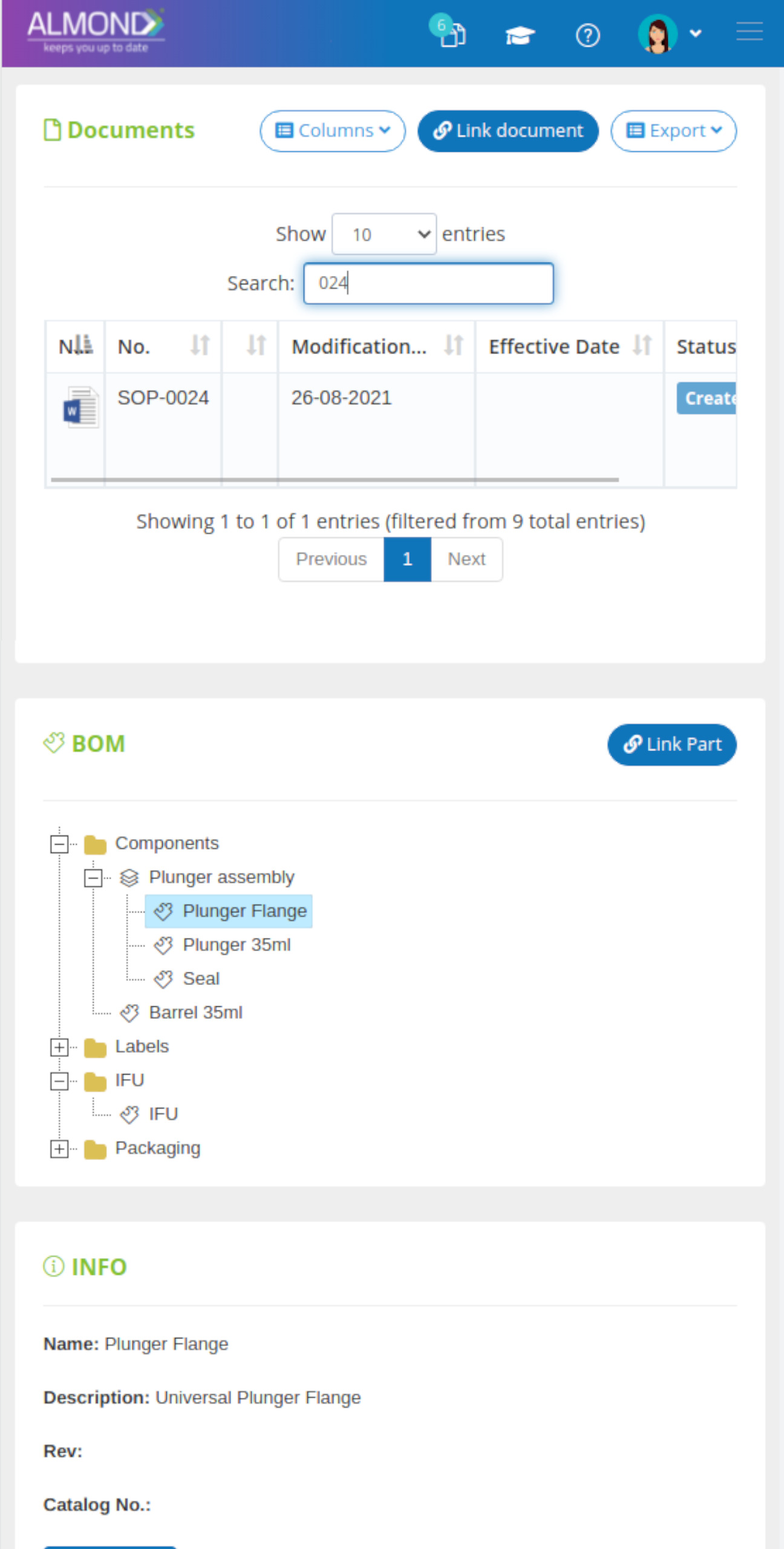
System Overview
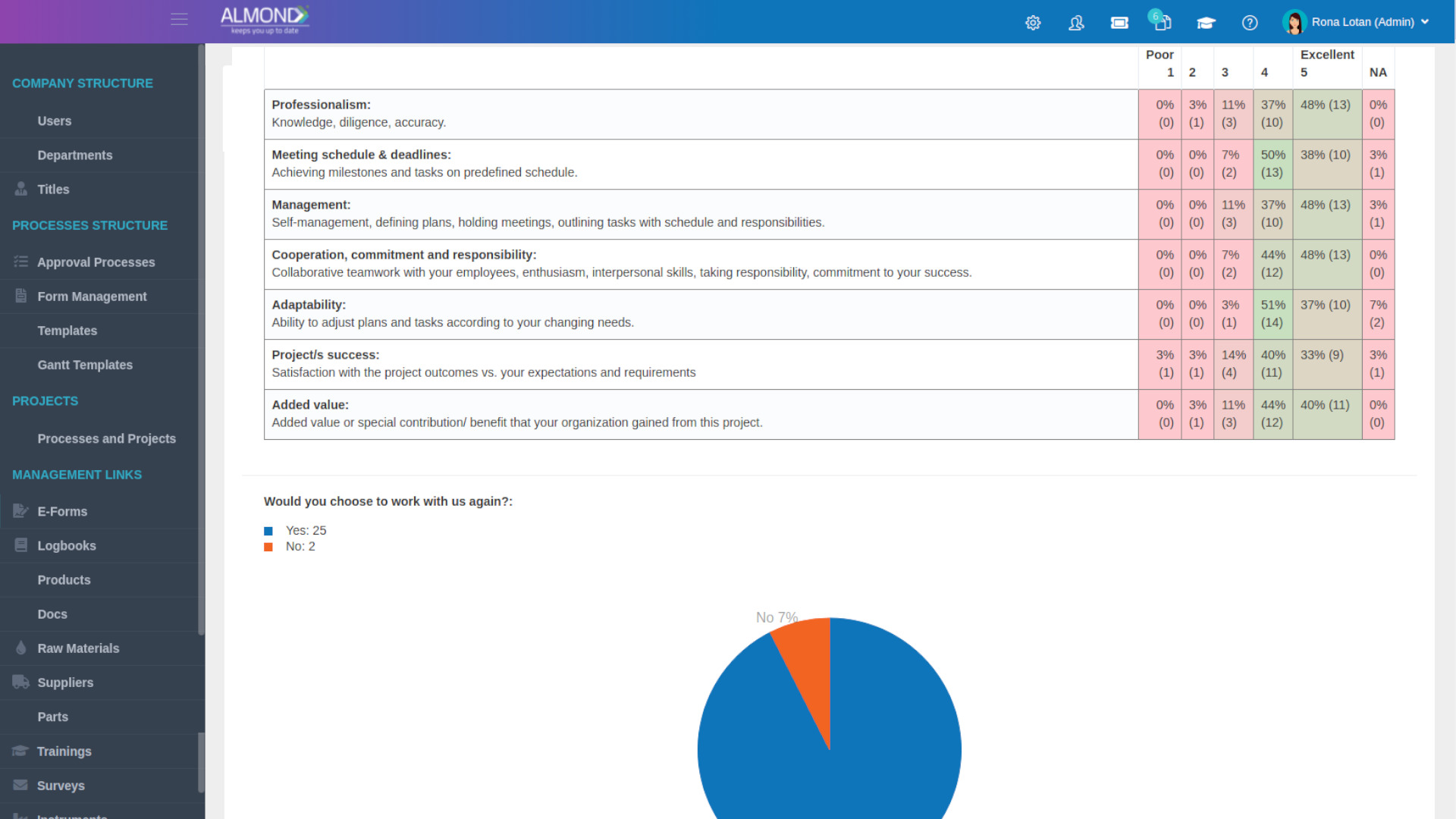
Customers
Our Customers Say

Almond was established from Gsap vast experience in the Biomed industry in order to accelerate and promote healthcare companies

Gsap Is An International Consulting Firm For Healthcare Companies, Providing End To End Services Throughout The Life Cycle Of Medical Products
Go To Company Website 
Almond Is A Professional, Cloud-Based, Quality Management Software (eQMS) For Healthcare Companies
About Us SE Pharma provides Validation and Calibration services along the supply chain of pharmaceuticals and Medical devices
Go To Company Website An innovative center for training and learning for Pharma, Advanced therapies, Medical Devices and Medical Cannabis Industries
Go To Company Website